Environmental Engineering Reference
In-Depth Information
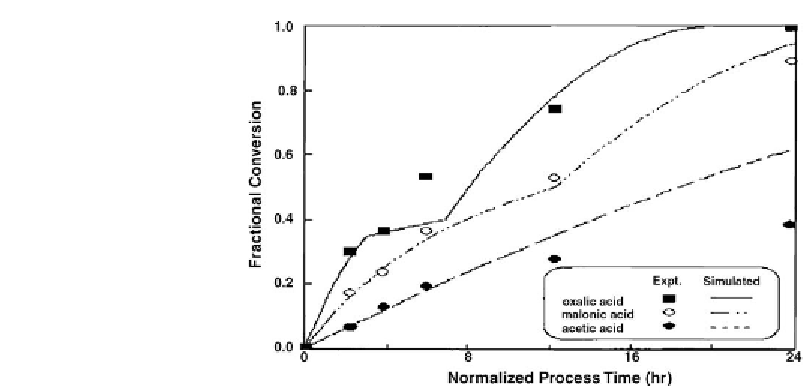
Figure 7.16: Comparison of experimental data with simulated data [From ref.
490
. Reprinted
with permission].
When the reaction rate is much faster than mass transfer, i.e.:
k
=
D
e
the rate is under diffusion control. Otherwise, it is under kinetic control.
In this set of equations,
r
and
R
represent radial coordinate of pores and pore radius,
respectively,
τ
D
and
τ
K
time in diffusion control and kinetic control, respectively,
ρ
B
density
and
C
A
concentration of leaching agent.
Normalized conversions determined experimentally during leaching V using 0.66M
concentration of oxalic acid, malonic acid, and acetic acid were compared with the values
predicted by the model. The results are shown in
Fig. 7.16 [490]
. The simulated values are
represented by lines, while experimental data are indicated by symbols.
7.1.3 Emissions from Rejuvenation by Organic Agents
The compliance with environmental regulations is a requirement before any process can be
commercialized. Apparently, for a rejuvenation process employing aqueous solutions of
organic agents, the control of emissions can be maintained by the established methods. Thus,
there is no need for development of any special techniques to suit the rejuvenation process.
Gaseous, liquid, and solid emissions are released to various degrees. Because of the
non-corrosive environment, the safety requirements during the rejuvenation using aqueous
solution of organic acids can be fulfilled without any difficulties. Moreover, most of the units
of rejuvenation process can be constructed from the readily available materials.