Environmental Engineering Reference
In-Depth Information
Figure 7.13: Chemically controlled vanadium removal from vanadium sulfide at 25
C (0.66M
oxalic acid) [From ref.
480
. Reprinted with permission].
proceeds in two stages. The change from stage 1 to stage 2 appears to be at a particular time
(e.g., 3 to 5 h). This depends on the type of leaching agent, which has an impact on both
kinetics and mass transfer coefficients. Therefore, a parameter such as effective diffusivity has
to be taken into consideration to describe leaching phenomena in more realistic terms. The
effective diffusivity (
D
e
) of a leaching agent can be determined using the following
approximation
[493]
:
ε
2
D
D
e
=
(7.6)
where
D
is the diffusion coefficient and
ε
is the catalyst porosity. It is evident from
Table 7.7
[490]
that effective diffusivities for acetic acid, malonic acid, and oxalic acid change with
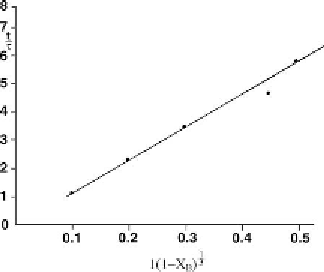
Figure 7.14: Diffusion controlled vanadium removal from spent catalyst at 25
C (0.66M oxalic
acid) [From ref.
480
. Reprinted with permission].