Environmental Engineering Reference
In-Depth Information
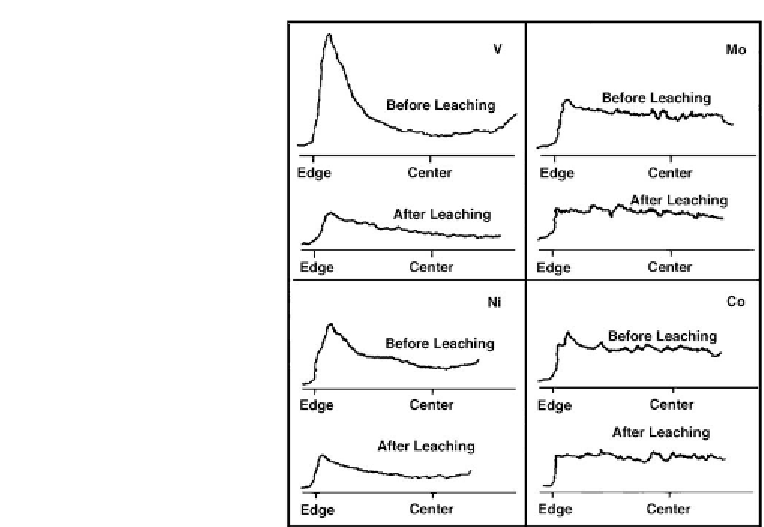
Figure 7.11: Effect of leaching on radial distribution of metals [From ref.
476
. Reprinted with
permission].
with the solution of oxalic acid
H
2
O
2
. Thus, after leaching, the distribution of the V and Ni
was significantly altered compared with little change in that of Co and Mo.
+
For coked catalyst, the metal oxidation began during the first contact of the catalyst surface
with the oxalic acid
H
2
O
2
mixture. At the same time, in the decoked catalyst, most of the
metals were already converted to an oxidic state during decoking. For coked catalysts, both
molecules of the acid and H
2
O
2
have to diffuse from the solution through the layer of coke to
reach the surface of particles. Among the metals present in spent catalysts, V will be oxidized
and leached out to the greatest extent compared with the other metals. Thus, the extensive
information in the literature confirmed that in spent catalysts, V is concentrated on the external
surfaces of particles as it is confirmed in
Fig. 7.11 [476]
. Under such conditions, a high
selectivity for leaching V is ensured. This is clearly confirmed in
Fig. 7.12 [484]
indicating
significantly enhanced selectivity for leaching V from coked catalyst compared with decoked
catalyst, i.e., for coked catalyst at about 80% V removal, the removal of Mo approached about
20% compared with more than 60% for decoked catalyst.
+
It is believed that there is an optimal ratio of the acid/H
2
O
2
giving the highest selectivity. The
optimum may be established experimentally. Moreover, the optimum depends on the origin of
organic acid and oxidizing agent. For example, a much greater enhancement in the selectivity