Environmental Engineering Reference
In-Depth Information
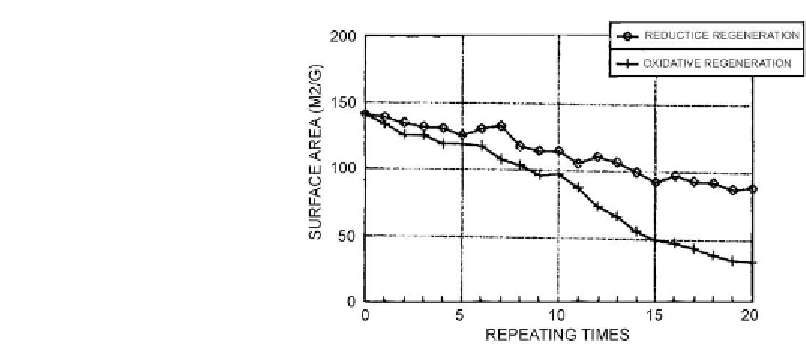
Figure 6.31: Surface area recovery during reductive and oxidative regeneration [From ref.
379
.
Reprinted with permission].
could be removed presumably via hydrogasification catalysed by active metals without having
an adverse effect on catalyst compared with the oxidative regeneration at 923 K. In the latter
case, sintering of the catalytically active phase could not be prevented. Moreover, at this
temperature, transformation of the
-Al
2
O
3
support to other forms of the Al
2
O
3
could become
quite evident. Therefore, comparison of the reductive regeneration with the oxidative
regeneration was not conducted under optimal conditions.
The coke structures in
Figs 4.20 and 4.21 [238,239]
may be used to illustrate difficulties,
which may be encountered during the reductive regeneration in H
2
. It is unlikely that such
structures (e.g., tetrahydrofuran insolubles [THFIS] after 6500 h in
Fig. 4.21
) could be
hydrogasified at a near atmospheric pressure of H
2
and temperatures at which the catalyst
stability can be still maintained. The increase in temperature and H
2
pressure to increase the
rate of hydrogasification of coke could have adverse effects on catalyst structure. Thus, at a
temperature suitable for coke removal, the catalyst over reduction could not be avoided. This
would be also accompanied by recrystallization of the catalytically active phases. Therefore,
the in-situ regeneration using reductive agents may not be feasible, particularly for the
catalysts used in severe hydroprocessing operations.
The spent hydroprocessing catalyst used for bitumen upgrading was reductively regenerated at
10MPa of H
2
in the presence of hydrogen donor solvents, such as tetralin and tetralin-pyrene
mixture
[439]
. These solvents were compared with gas oil. The following order in the
effectiveness of coke removal was established: gas oil
<
tetralin
<
tetralin-pyrene. The highest
effectiveness of tetralin-pyrene mixture was attributed to the ability of pyrene to form very
active H-donors, such as hydropyrenes. The HDS activity of the regenerated catalyst was
significantly enhanced.