Environmental Engineering Reference
In-Depth Information
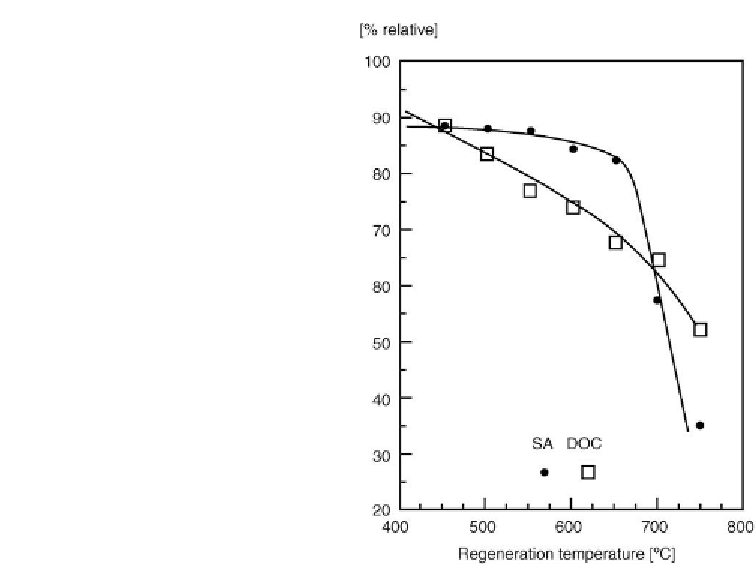
Figure 6.22: Effect of regeneration temperature on recovery of surface area (SA) and on dynamic
O
2
chemisorption (DOC) [From refs
367
and
368
. Reprinted with permission].
The usefulness of the O
2
chemisorption method was also confirmed by Cable et al.
[406]
.In
agreement with
Fig. 6.18 [401]
, the abrupt surface area decline above 600
◦
C was rather
evident. At the same time, the decline in the DOC was more gradual. Nevertheless, only
regeneration below 500
◦
C ensured a desirable level of the activity recovery. The DOC values
were consistently lower than corresponding surface area values suggesting that a high recovery
of surface area does not ensure a high recovery of activity. However, these observations cannot
be generalized. For example, Inoguchi et al.
[407]
reported that a complete recovery of both
surface area and activity was achieved for a spent catalyst deactivated only by coke, whereas in
the presence of contaminant metals neither surface area nor activity of the fresh catalyst could
be restored on regeneration. Another set of interesting data was published by Sakabe and Yagi
[408]
who studied regeneration of the spent CoMo/Al
2
O
3
catalyst used for hydroprocessing of
a residue. For this catalyst, a loss in the HDS activity on regeneration was observed. At the
same time, the activity of the catalyst for hydrocracking reactions improved. Similar
observation was made by Cable et al.
[406]
. Then, the regenerated catalyst may be used in an
operation requiring a high hydrocracking service, although this observation cannot be
generalized.