Environmental Engineering Reference
In-Depth Information
underestimate of the rate constants. This was particularly evident for the catalyst that was not
presulfided prior to hydroprocessing test. As it was indicated earlier, reliable values of the
kinetic parameters can only be obtained from the determination of products with time on
stream. These values are much higher than those from the TGA tests. This may be attributed to
the turbulent flow present in the fixed bed reactor compared with the laminar flow present in
the TGA system. The former ensured more efficient contact of O
2
with spent catalyst. Also,
the rate constants for sulfided catalyst were consistently higher than those for the oxidic
catalyst. Most likely, this was caused by the difference in reactivity of coke. Thus, because of a
higher hydrogenation activity, the coke on sulfided catalyst should be less refractory, i.e.,
softer, compared with the coke on the oxidic catalyst. This was supported by the higher H/C
ratio of coke on the former spent catalyst.
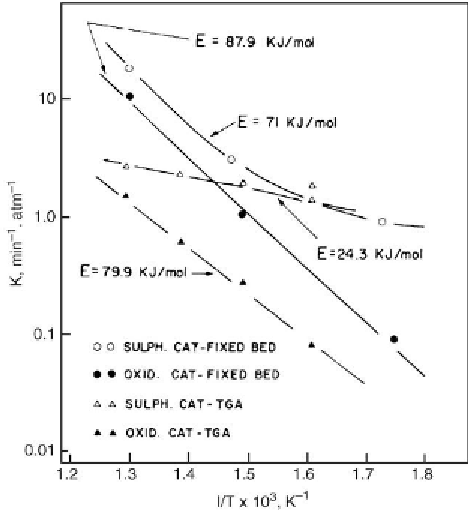
The rate constants in
Table 6.3 [387]
were expressed in the form of Arrhenius plots (
Fig. 6.14
)
[391]
for the estimate of activation energies. It should be noted that these rate constants were
determined during very early stages of burn-off, i.e., during less than 50 s. Therefore, they
represent chemically controlled burn unaffected by diffusion. Activation energies for the
oxidic catalyst were consistently higher than those for the sulfided catalyst. For the latter
catalyst, the activation energy obtained from TGA results was affected by the weight loss
Figure 6.14: Log k versus 1/
T
correlations for burn-off performed in fixed bed and TGA reactors
[From ref.
391
. Reprinted with permission].