Environmental Engineering Reference
In-Depth Information
formation during the exposure of sulfided catalysts to air cannot be ruled out. The sulfate
formation during the exposure to air was also postulated in
Chapter 5
for spent catalysts.
A significant complexity of the mechanism of the oxidative regeneration of spent catalysts
should be noted. This results from rather complex nature of coke and metals, which deposit on
catalyst surface during the operation. Thus, only recently, the chemical structure of such coke
was described in more details
[238,239]
. The interaction of coke with catalyst surface during
regeneration is an important part of the overall mechanism. Because of the structural
complexity and continuous change, the involvement of the inorganic part of spent catalysts
during regeneration needs to be further investigated. Therefore, some assumptions and/or even
speculation while discussing the mechanistic aspects of the oxidative regeneration of spent
hydroprocessing catalysts could not be avoided.
6.2.2 Kinetics of Oxidative Regeneration
The estimate of kinetic parameters may be desirable to obtain a more quantitative picture of
regeneration process. There are several methods that can be used for quantifying the removal
of coke during regeneration. The method based on the change in conversion with time on
stream requires several burn-off experiments performed under the same conditions but at
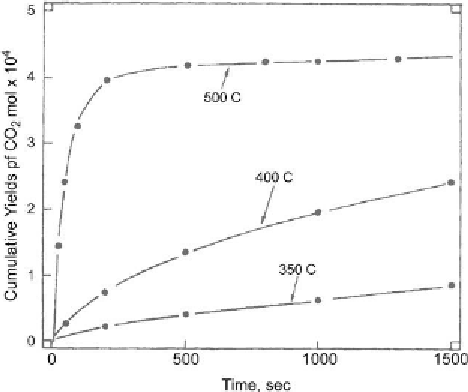
different duration. Apparently, the most suitable method involves the quantitative on-line
analysis of all burn-off products in the course of the experiments. An example of such results
is shown in
Fig. 6.11 [374]
correlating cumulative yields of CO
2
with time at different
Figure 6.11: Cumulative yields of CO
2
from isothermal burn off of spent catalyst in air [From
ref.
374
. Reprinted with permission].