Environmental Engineering Reference
In-Depth Information
charge
Z
of the Coulomb center is
J
n
Z
2
e
2
/(
a
0
n
2
). From this we have for the
decrease
Δ
J
in the atomic ionization potential in a plasma [74]
Ze
2
N
1/3
i
Δ
J
D
J
n
.
(1.102)
In particular, according to calculations by the method of molecular dynamics, this
value is
3.2
Ze
2
N
1/3
i
Δ
J
D
.
(1.103)
One can consider the ionization potential decrease for atoms in a plasma to be a
result of the action of plasma microfields. Indeed, let us consider an excited elec-
tron located in the field of the Coulomb center with charge
Z
and an external elec-
tric field of strength
E
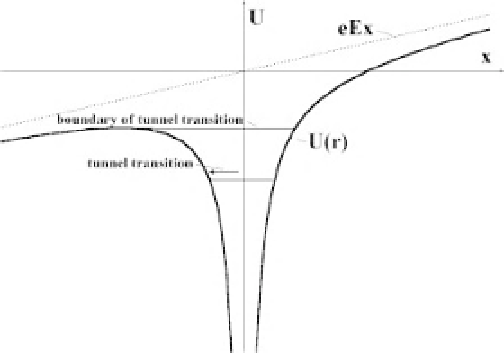
. Then the interaction potential
U
between an electron and
the Coulomb center in the direction
x
oftheelectricfieldis(seeFigure1.6)
Ze
2
x
U
D
eEx
,
where the origin of the frame of reference is taken as the Coulomb center. The
maximum of this interaction potential
U
max
gives the decrease of the ionization
potential:
2
p
eE
Δ
J
D
U
max
D
Ze
2
.
In particular, if we use a typical electric field strength in a plasma in accordance
with (1.26),
E
0
2.6
ZeN
2/3
i
D
, we obtain for the decrease in the ionization potential
3.2
Ze
2
N
1/
i
in accordance with (1.103).
Thus, the presence of microfields in a plasma may lead to the disappearance of
excited atom states in a plasma. The disappearance of spectral lines was observed
for the first time by Lanchos [75, 76] in 1930, and this effect means that the radia-
tive lifetime for an observed atomic transition becomes equal to the time for the
Δ
J
D
Figure 1.6
The potential energy in the plane of the electric field for an electron located in the
field of a Coulomb center and electric field.