Environmental Engineering Reference
In-Depth Information
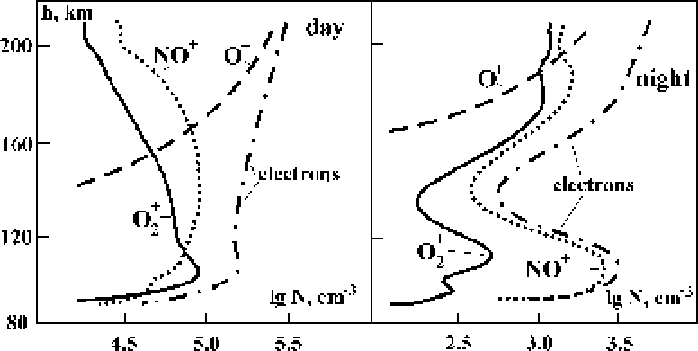
tions, and Figure 6.22 gives the daytime and nighttime distributions for the num-
ber densities of electrons and basic ionospheric ions according to evaluations [148].
The next higher layer of the ionosphere, called the E-layer, is at an altitude from
90 to 140 km. Charged particles of the E layer are formed by photoionization of air
caused by solar UV radiation. These charged particles drift to lower layers of the
atmosphere and are the source of plasma in the D layer. The electron number den-
sity in the E layer of the ionosphere is of the order of 10
5
cm
3
.Negativeionsare
nearly nonexistent in this layer, and the basic types of positive ions are NO
C
and
O
2
. The decay of charged particles in the E layer is due to dissociative recombina-
tion of electrons and molecular ions, or to transport of charged particles to lower
layers of the atmosphere.
One can estimate a background altitude between D and E layers from the relation
N
e
KN
e
[O
2
]
2
,
α
where
is the dominant recombination process from processes 13-15 in Table 6.6,
and
K
is the rate constant for three body process 21 in Table 6.6. This estimate
means that in the E layer electrons are lost as a result of dissociative recombina-
tion, whereas in the D layer they attach to oxygen molecules, and then negative
ions of various kinds determine the negative charge of the ionosphere. Using the
values of the rate constants given in Table 6.6 and using in the above formula
N
e
α
10
5
cm
3
, we find the boundary between the D and E layers corresponds to
the number density of oxygen molecules [O
2
]
10
14
cm
3
, which corresponds to
an altitude of 90 km.
A higher ionospheric layer (the F
1
layer) is located at an altitude of 140-200 km.
Above it (up to an altitude of about 400 km) is the F
2
layer of the ionosphere. The
electron number density in these F layers is 10
5
-10
6
cm
3
.Thebasictypeofposi-
tive ions is O
C
. Charged particles of the F layers of the ionosphere are formed by
photoionization of atmospheric oxygen (the basic component of the atmosphere at
these altitudes) by solar radiation. Loss of electrons from these layers is caused by
Figure 6.22
The average altitude distributions of electrons and basic positive ions in the iono-
sphere according to evaluations [148].