Environmental Engineering Reference
In-Depth Information
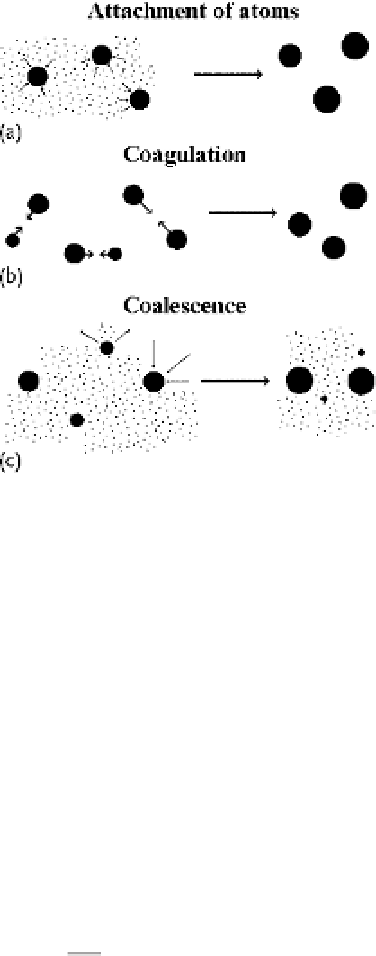
Figure 6.18
The mechanisms of cluster
growth: (a) attachment of free atoms to clus-
ters; (b) coagulation resulting in joining of
clusters on contact; (c) coalescence that pro-
ceeds owing to equilibrium between the clus-
ter and the parent vapor. As a result of atom
attachment to clusters and cluster evapo-
ration, small clusters decompose and large
clusters grow. As a result, the average cluster
size increases.
atoms compared with that of bound atoms
N
b
,thatis,
N
N
b
.Anothermecha-
nism of cluster growth, given in Figure 6.18c, is known as Ostwald ripening [116]
or coalescence and takes place at such temperatures where for small clusters the
rate of evaporation exceeds the rate of atom attachment, and for large ones we have
the opposite relation between these rates. Then the equilibrium between clusters
and their atomic vapor leads to evaporation of small clusters and growth of large
clusters. As a result, the average cluster size increases.
The size distribution function
f
n
for clusters in the coagulation process is de-
scribed by the Smoluchowski equation [117]:
f
n
Z
k
(
n
,
m
)
f
m
dm
Z
k
(
n
@
f
n
@
1
2
t
D
C
m
,
m
)
f
n
m
f
m
dm
.
(6.76)
Here
k
(
n
m
,
m
) is the rate constant for process (6.75), the factor 1/2 accounts for
the fact that collisions of clusters consisting of
n
m
and
m
atoms are present in
the equation twice, and the distribution function is normalized as
Z
f
n
dn
D
N
cl
,
where
N
cl
is the number density of clusters. Note that the conservation of the total
number density
N
b
of bound atoms in clusters in the course of the cluster growth
process follows from the Smoluchowski equation (6.76). Indeed, multiplication of