Environmental Engineering Reference
In-Depth Information
than that according to the Boltzmann formula (1.43), as it is given by (3.66), and
the rate constant for stepwise ionization is lower than that given by (3.69). We
analyze this in a heliumplasma, being guided by the electron number density
N
e
D
10
14
cm
3
. The number density of electrons in glow and arc discharges may
be included in this interval, and the indicated electron number density provides the
regime of electron kinetics with dominant collisions between electrons that allows
one to operate with the electron temperature
T
e
.
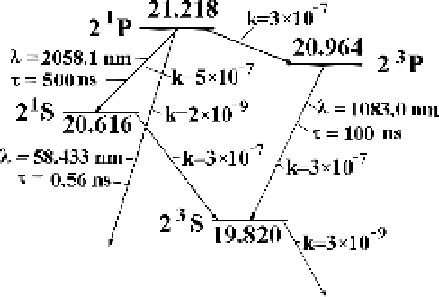
We restrict ourselves to four excited states of the helium atom, He(2
3
S
), He(2
1
S
),
He(2
3
P
), and He(2
1
P
), and include them in the scheme of kinetics for excited
states. The excitation energies with respect to the ground state energy are given
in electronvolts in Figure 3.10. The parameters of radiative transitions are taken
from Table 2.8 [51, 52]. The quenching rate constants resulting from electron im-
pact are expressed in cubic centimeters per second and these values for resonantly
excited states are given in Table 2.8, whereas the rate constant for quenching of
the 2
3
S
state is taken from Table 2.9 and the measured rate constant [53] for the
transition
e
10
11
He(2
1
S
)
He(2
3
S
) is used. Next, considering the transition
C
!
e
C
He(2
3
P
) proceeds from exchange between incident and bound
electrons, we use the cross section of electron exchange [54] in evaluating the rate
constant for this process. One can express the rate constants for inverse processes
through these rate constants on the basis of the principle of detailed balance (2.57).
Thus, in this way, ignoring higher excited states, we have all the information about
the kinetics of these excited states, although with restricted accuracy. Solution of
balance equations for the number density of atoms in these excited states allows
us to have the populations of these states at different electron temperatures and
electron number densities in the stationary case.
Because our goal is to obtain the qualitative character of kinetics of excited states
in this case, we shall perform a rough analysis of the population of excited states in
this case. For the resonantly excited state He(2
1
P
), (3.66) in the indicated range of
He(2
1
P
)
e
C
!
e
C
Figure 3.10
The lowest excited states of the
helium atom. The energy of state excitation
is expressed in electronvolts, and quantum
numbers of states are given within the frame-
work of the
LS
coupling scheme. The wave-
lengths of radiative transitions are indicated
in nanometers and the radiative lifetimes of
states are represented in nanoseconds.