Environmental Engineering Reference
In-Depth Information
since
dN
/
dt
is the energy that is consumed in atom excitation by electrons,
and
eEw
e
N
e
is the energy that is transmitted to electrons from the electric field.
On the basis of the above formula for the excitation rate, we have for the excitation
efficiency
Δ
ε
Δ
ε
T
ef
exp
3/2
4
3
p
π
Δ
ε
T
ef
D
,
Δ
ε
T
ef
.
(3.56)
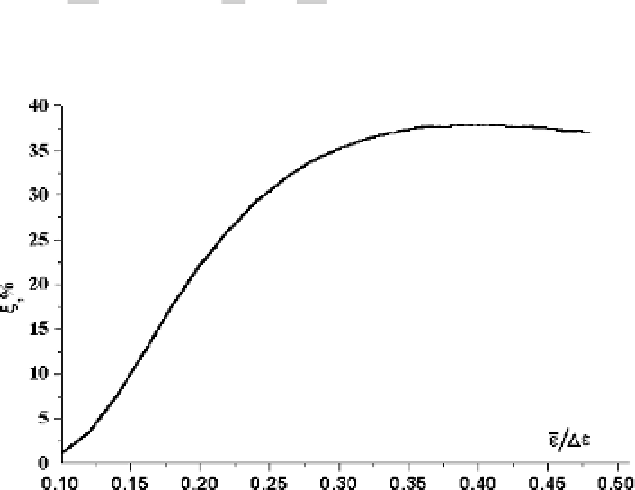
Figure 3.7 shows the dependence of the efficiency of atom excitation
on the ef-
fective electron temperature under given conditions.
We represented above two limiting cases for atom excitation in an ionized gas. In
reality we have several parameters whose combination leads to the corresponding
limiting cases. These parameters are
m
e
M
,
T
ef
Δ
ε
g
ν
q
μ
D
γ
D
,
δ
D
.
(3.57)
g
0
ν
This means that we have additional limiting cases with respect to those given
by (3.52) and (3.54). We now consider one more limiting case, when, on the one
hand, the rate of excitation is expressed through the rate constant of excitation and,
on the other hand, the distribution function varies significantly due to the exci-
tation process near its threshold. As before, we assume the excitation threshold
corresponds to the tail of the energy distribution function, that is,
1. We ac-
count for the excitation process by inserting into the right-hand side of (3.53) the
term
γ
<
ex
is the excitation rate, and this equation near the excitation
threshold takes the form
@
ν
ex
f
0
,where
ν
T
ef
@
f
0
f
0
@
2
m
e
M
ν
Δ
ε
@
@
ε
f
0
@
ε
C
t
C
ν
ex
f
0
D
0,
and below the excitation threshold this equation coincides with (3.53).
Figure 3.7
The efficiency of atom excitation in a plasma in a constant electric field as a func-
tion of the reduced average electron energy in the case when the rate of electron-atom elastic
collisions is independent of the electron velocity.