Chemistry Reference
In-Depth Information
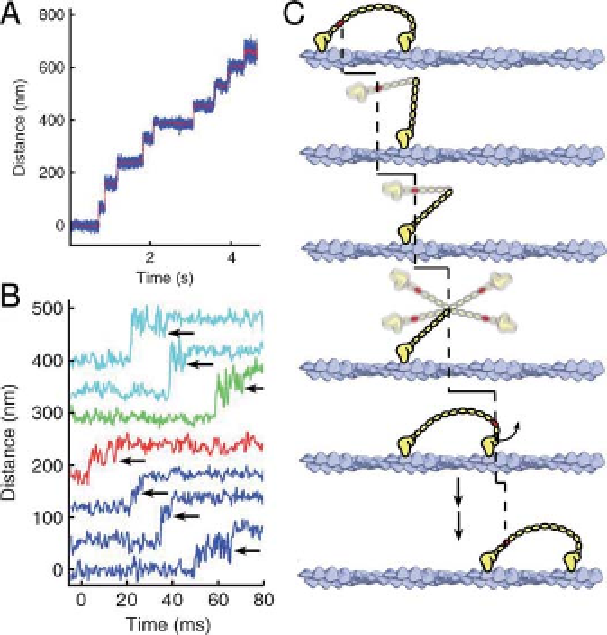
Figure 3.12 A myosin V dimer is labeled with a
gold nanoparticle on one of its lever arms and is
located by darkfield microscopy. A, data trace
with a 40 nm gold particle, 3 mM ATP. Frame
interval: 0.32 ms. The red trace represents low-
pass filtered DIONA. B, 49-nm substeps. The
ends of the substeps are indicated by arrows.
Note the increase in variance during the substep,
suggesting single-headed attachment. C,
cartoon model that explains the results;
increased variance during the step is the
thermal search. From Ref. [36].
the myosin-ADP-Pi
i
state binds to actin, the Pi
i
is released quickly and nearly
irreversibly [81], capturing the distortion in the molecule produced by the random
process.
Why does the trailing head detach before the leading head? The high processivity of
the motor requires that the biochemical cycles of the two heads be kept out of
synchrony. This feature ensures that the trailing head is the one to step forward and it
also prevents both heads from detaching at the same time. The difference between
the stroke size (24 nm) and the step size (36 nm), and the extra reach delivered by the
diffusional search imply that when both heads are bound to actin, the molecule is
under internal mechanical strain. Each head pulls the other one toward the center of
themolecule. Due to the slow kinetics of ADP release, both heads have ADP bound in
thewaiting state between steps. If the backward strain on the leading head suppresses
ADP release and/or the forward strain on the trailing head accelerates ADP release,
then the trailing head will release its ADP
first, ATP will bind rapidly to the trailing
head inducing detachment and initiating a forward step [63, 68, 70, 82]. Thus
the intramolecular strain generated by the thermal
fluctuations is trapped by the