Chemistry Reference
In-Depth Information
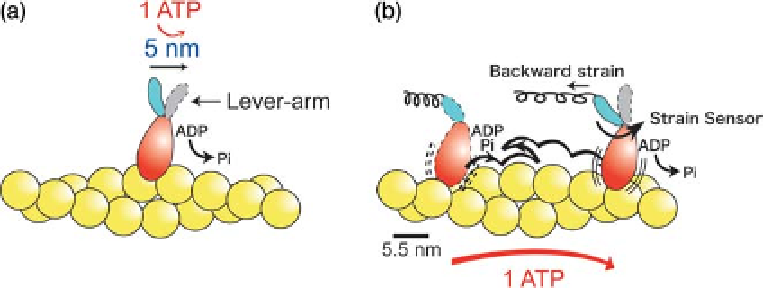
Figure 2.9 Lever arm model and bias Brownian
model. (a) Lever armmodel. The displacement is
developed by a conformational change (lever
arm tilting), tightly coupled to the ATP hydrolysis
cycle. (b) Bias Brownian model. The myosin
head (ADP-Pi) undergoes rapid attachment and
detachment cycles with an actin filament in the
weakly binding state. The Brownian motion is
biased in one direction according to the potential
slope along the actin helix (see Figure 2.8a). The
neck domain of the myosin head acts as a strain
sensor that controls the transition from the weak
to strong binding state, coupled with Pi
release [79]. When the myosin head approaches
the forward actin target by Brownian motion, the
neck domain is pulled backward and the strain
sensor is then switched on to ensure that the
myosin head is strongly bound to the actin. The
conformational changes in the neck domain are
coupled to the action of a strain sensor which
may cause isometric force.
site, bends. In the early 1990s, the crystal structure [58] of the myosin head was
elucidated showing that the neck domain which is attached to the motor domain of
the head, changed its angle relative to themotor domain depending on the formof the
bound nucleotide. Based on these
findings, the cross-bridge swinging model has
been re
ned to the lever-arm swingingmodel. Here the neck domain acts as a lever
arm and a small conformational change in the motor domain causes the lever arm to
swing resulting in large displacements of 5 to 10 nm (Figure 2.9a) [59, 60]. Many
studies have agreed that the neck domain changes its angle during both muscle
contraction [60, 61] and in vitro motility assay [62]. The observed conformational
changesmay contribute to the generation of isometric force at large loads but may not
cause the sliding movement at smaller loads. In our bias Brownian step model
(Figure 2.9b) the conformational change in the neck domain coupled with Pi release
is not the direct cause of the swingmovement. The lever armmodel hypothesizes that
the neck domain swings parallel to the longitudinal axis of the actin
filament to
directly produce displacements in the forward direction. However, several studies
using electron microscopy have suggested that the direction of the neck domain
swing is not parallel but diagonal to the longitudinal axis of the actin
filament [54, 63].
Therefore, the conformational changes in the neck domain coupled to Pi release may
cause rotation of the actin
filament, consistent with our model (Figure 2.9b).
Several studies using optical trapping nanometry have reported that the displace-
ments by chemically-modi
ed and genetically-engineered myosin heads with various
neck domain lengths are approximately proportional to the neck lengths [64
-
66],
consistent with the lever arm swinging model [59, 60]. As shown in our model
(Figure 2.9b), the displacements we observed depended on the interacting length