Chemistry Reference
In-Depth Information
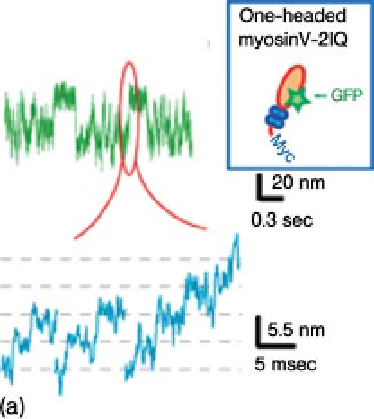
Figure 2.7 Sub-steps within a displacement of
myosin-V [35]. (a) One-headed myosin-V with
short (2IQ) neck. (b) One-headed myosin-V with
long (6IQ) neck. (c) two-headed myosin-V with
long (6IQ) necks. (d) The Hopping model for
myosin-V stepping. The rear head searches for
the actin target in the forward direction by a
hopping movement on the actin subunits
according to a potential slope along the actin
helix. Black closed circle¼ATP or ADP-Pi.
F
19 nm
indicates displacements by neck bending and its
diffusion, and the dark gray band of
¼
Force. The light gray band of
17 nm
indicates directional steps on actin monomers
according to the potential slope along the actin
helix.
36-nm step was observed. Such intermediate steps have been previously observed
using optical trapping nanometry at high loads [32] or in the presence of 2,3-
butanedione monoxime [36]. In this experiment, the intermediate step was clearly
observed even at low loads. It is likely that the intermediate step was also slowed by
attaching the two-headed myosin-V to a large scanning probe. Figure 2.7c shows the
rising phases of the intermediate steps on an expanded time scale. The sub-steps of