Chemistry Reference
In-Depth Information
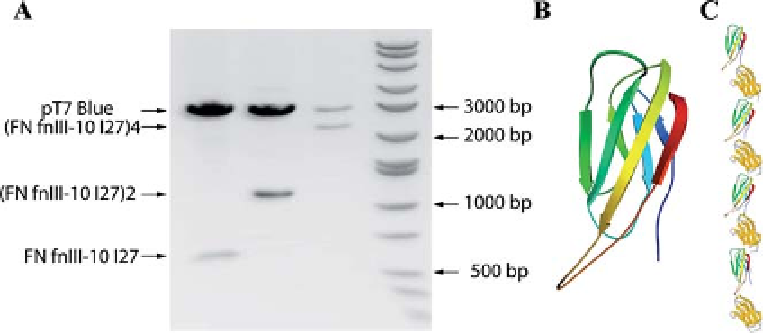
Figure 13.1 Construction of
10
FNIII fibronectin
polyprotein. (A) Agarose gel stained with
ethidium bromide showing cDNAs of the
digestedmonomer construct (
10
FNIII-I27) in the
first lane, the dimer in the second lane
(
10
FNIII-I27)
2
and the tetramer in the third lane
(
10
FNIII-I27)
4
. The top band always shows the
pT7 Blue vector. The last lane shows the size
standard. (B) Diagram of the
-sandwich
structure of the
10
FNIII fibronectin module.
(C) Schematic representation of the final
polyprotein (
10
FNIII-I27)
4
.
b
proteins. The
rst force spectroscopy AFMexperiments were performed using single
proteins [30], however, these experiments lacked any
ngerprint for an unambiguous
unfolding event. This problem was solved by ligating multiple copies of a single
protein module and expressing the resultant gene in bacteria (Figure 13.1). The
engineering of proteins made from tandem repeats of an identical module, poly-
proteins, has then permitted a module-by-module investigation of the mechanical
properties of native proteins. Polyproteins provide a consistent
fingerprint, which
then allows us to identify the molecule of interest from other background interac-
tions [31]. Furthermore, the construction of engineered polyproteins makes it
possible to carry out extensive mutagenesis experiments [32
-
34]. More recently, a
simpler approach has been demonstrated where pairs of cysteine residues are
introduced by mutagenesis at various locations throughout the protein structure,
thereafter, polyproteins are simply obtained through the spontaneous oxidation of
the cysteine residues between protein monomers [35, 36]. There are many modular
proteins that perform their function in tandem, such as the immunoglobulin
modules in the muscle protein titin [37] or multiple ubiquitin modules in protein
degradation [38]. While the use of polyproteins over the past 8 years has permitted the
rapid development of single-protein AFM techniques, their use is not without
controversy [39
-
42] and ideally, the single-protein AFM techniques will evolve to
a point where the use of protein monomers can be reliably recorded.
13.2.1
Force-extension Spectroscopy
In a force extension experiment a single polyprotein is stretched between the tip of a
cantilever and a
flat substrate (gold) that is mounted on a piezoelectric positioner