Chemistry Reference
In-Depth Information
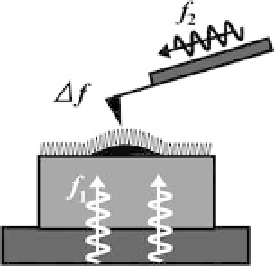
Figure 12.8 Schematic of proposed high-speed
ncAFM set-up. Ultrasonic waves are launched
from the bottom of the sample as well as from
the cantilever. The frequencies, f
1
and f
2
, are
much higher than the fundamental resonant
frequency, f
c
, of the cantilever, and their
difference,
ultrasonic waves interfere with each other and
produce acoustic waves with a frequency of
f,
which excites the cantilever. The wave front of
ultrasonic waves with frequency f
1
, is formed at
the sample surface and sensed by the cantilever
which is not in contact with the surface but is
close to it.
D
D
f
| f
1
f
2
|, is similar to f
c
. The two
When the sample in a solution consists only of protein molecules attached to a
uniform substratum, the wave-front of the ultrasound propagated through the
substratum may trace the sample topography. This wave front can probably be
detected by the cantilever tip, which is close to but not in contact with the sample
surface.
A second possibility for high-speed, non-contact imaging may derive from ion-
conductance scanning probe microscopy (ICSPM), which has already satis
ed the
non-contact condition [45]. Due to the progress in fabrication techniques to produce
very sharp glass capillaries with a small pore at the end, the spatial resolution has
reached a few nm [46]. Immobile protein molecules of
14 nm on living cell
membranes have been successfully imaged [47]. However, in order to materialize
high-speed ICSPM, we need to
find a method to increase the bandwidth of ion-
conductance detection because ionic currents through a small pore are very low.
Is high-speed recognition imaging even possible? The effective concentration of a
probemolecule attached to a cantilever tip depends on the tether length. With a tether
length of 2 nm, the concentration becomes
50mM, which is high enough for the
association reaction to take place in 20
m
s for a system with a typical association rate
10
6
M
1
s
1
. This suggests that recognition imaging at a moderate
constant of 1
rate (
0.1 s/frame) is possible, so long as the cantilever oscillation amplitude is small.
At present, we have no technology that allows us to study the structural dynamics of
intracellular organelles at high spatial and temporal resolution. Recently, a far-
eld
fluorescence imaging technique (STED microscopy) with diffraction-unlimited
resolution has been developed based on stimulated emission depletion of
uor-
ophores [48, 49]. Its high spatial resolution has been demonstrated by resolving the
arrangements of densely packed 40-nm beads and supramolecular aggregates in a
cell membrane. However, it is unlikely that this new microscopy will attain a high
temporal resolution without sacri
cing the spatial resolution because of the limited
number of photons collected in a short time bin. The recently demonstrated