Chemistry Reference
In-Depth Information
dynamic PID controller, microtubules were destroyed. Similar results were seen
with actin and myosin. With the dynamic PID control, actin
filaments gliding on a
mica surface densely coated with myosin V were captured on video
(Figure 12.4d) [4, 12], whereas a low myosin V density resulted in minimal
observation of the gliding movement.
With an improved dynamic PID controller together with a newly-developed
optical de
ection sensor with low noise, the set point could be set at
>
0.9 and
thus actin
filaments gliding on a surface sparsely coated with myosin V were
successfully imaged [4, 12]. By chance, a short actin
filament entered the observed
region. Its entire length was within the region allowing all myosin V molecules
interacting with this
filament to be identi
ed. The interacting myosin V heads were
oriented in one direction, similar to the well-known arrow-head structure in
muscles. From this oriented structure, the polarity of the actin
filament was
identi
ed. The
filament moved towards the minus (pointed) end, which was the
natural direction (Figure 12.5). However, conformational changes in the interacting
myosin V heads were not evident during the unidirectional movement of the
filament.
Recently, a prototype high-speed AFM, an improved version of our
rst
design [2, 3], has become commercially available (Nano Live Vision
manufactured
by Olympus and distributed by RIBM). Its users recently
filmed the ATP-
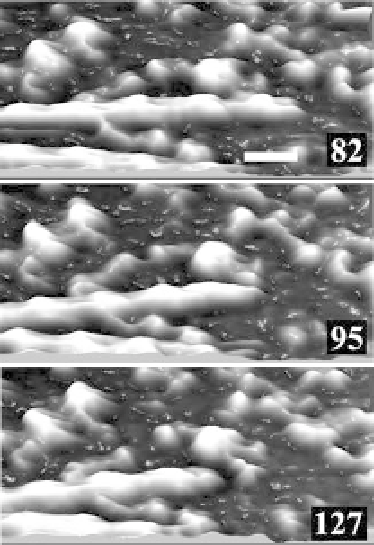
Figure 12.5 Three-dimensional images of actin filament sliding
movement captured by high-speed AFM. The number on each
image indicates the frame number. Scale bar 30 nm, imaging rate,
180ms/frame.