Chemistry Reference
In-Depth Information
The rates of conformer transitions, k
I ! II
and k
II ! I
, averaged over several
hundred transitions, strongly depend on the type and concentration of metal ions.
Figure 11.5C shows that (k
I! II
k
II ! I)
) increases with de creasing metal ion
concentration for Na
þ
,Mg
2 þ
and hexaminecobalt [131]. These rates were obtained
via cross-correlation analysis [31] as described in detail in the introduction to this
revised application. Higher valence ions make the transitions slower at the same
concentration. These observations suggest that the open structure, stable in the
absence of metal ions, may be an intermediate in conformer transitions. Metal ions
stabilize the stacked structures and HJ opening (or unfolding) necessary for the
conformer transitions, and tend to be less frequent at higher cation concentrations.
The characteristics of HJ conformer dynamics such as a relative bias between two
conformers are essentially the same in Mg
2 þ
concentrations ranging from sub-
millimolar (physiological free Mg
2 þ
level) to 50mM, except for changes in the
absolute rates.
þ
11.4.2.1 Single-molecule Three-color FRET on HJ
Since regular smFRET cannot obtain information on more than one inter-dye
distance at a time, we developed three-color FRET using the HJ as a model
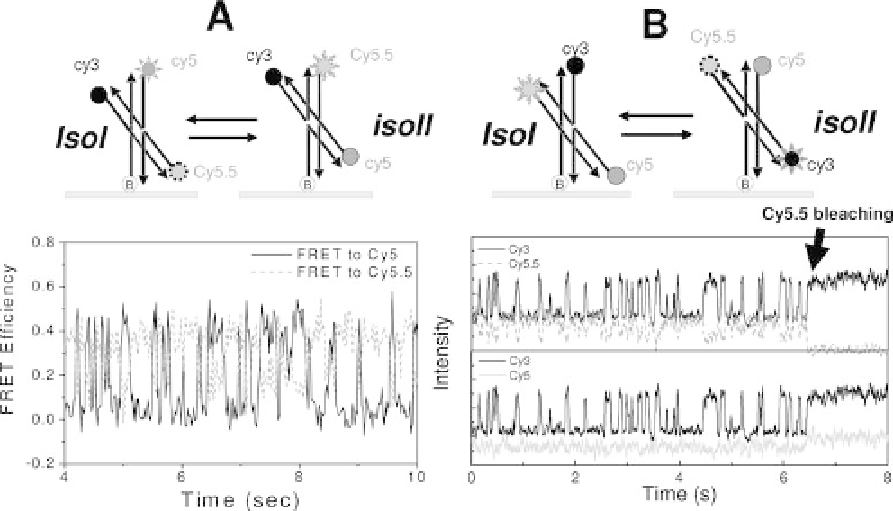
system [132]. First, an alternative acceptor (Cy5.5) was attached to the free arm of
junction 1 (helix R in Figure 11.4) so that the donor would transfer energy
alternatively to Cy5 or Cy5.5 upon conformer transitions (Figure 11.6A). With a
judicious choice of
uorescent
filters and careful correction for crosstalk between
Figure 11.6 First demonstration of three-color FRET.
Conformational transitions of Holliday junction were seen as
alternating FRET to two distinct acceptors. Adapted from [132].