Chemistry Reference
In-Depth Information
Figure 10.2 A crystal structure for the
a
3
b
3
g
associated with the
subunit. The noncatalytic
nucleotide-binding site on the
b
subcomplex of F
1
. The
subunits are
shown in yellow, green, and red, respectively. The
catalytic sites are located at the interface between
the
a
,
b
, and
g
subunit is
located at the opposite interface to the
a
b
subunit.
Three
subunits bind ANPPNP (non-
hydrolyzing ATP analog). Each of the three
a
subunits (arrows); however, most of
the residues responsible for catalysis are
a
and
b
b
subunit binds ANPPNP, ADP, or none.
Boyers novel idea did not gain much support until John Walker and colleagues
revealed the crystal structure of the
subcomplex of F
1
from bovine mitochon-
dria in 1994 (Figure 10.2) [6]. As mentioned above, in this crystal structure, the
a
3
b
g
3
a
and
b
subunits are alternately arranged and form a ring. A signi
cant feature of this
structure was the conformations of the three
b
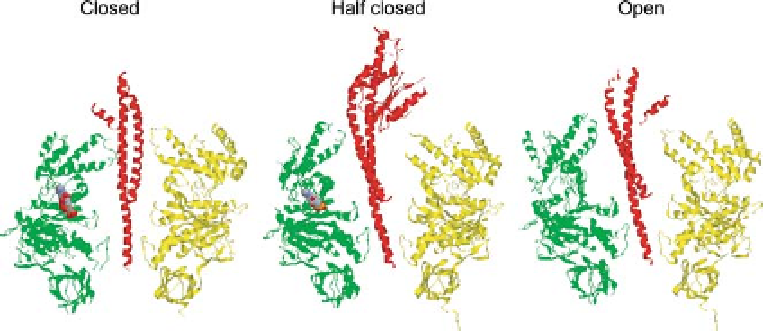
subunits (Figure 10.3). Each of the
Figure 10.3 Three different conformations of the
b
bind nucleotide are shown in closed
conformation (left). In the 2001 structure, one
subunit. One of the three
a
and
b
subunits and
b
the
subunit are shown in yellow, green, and red,
respectively. In the 1994 structure, one
g
subunit that corresponds to the
subunit
without nucleotide in the 1994 structure, binds
ADP and sulfate and is shown in half closed
conformation (center).
b
subunit
that does not bind nucleotide is shown in open
conformation (right), and two
b
b
subunits that