Chemistry Reference
In-Depth Information
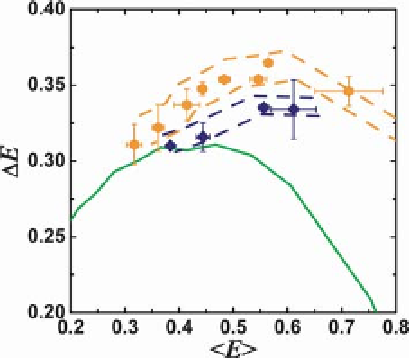
Figure 9.7 Effects of denaturant concentration
on distance distributions of unfolded CI2 (blue)
and ACBP (orange). The width of the E
distribution
the measurements for the unfolded proteins are
at or above the Gaussian chain limit (green line).
For each data set (CI2 or ACBP), lowering the
denaturant concentration results in
measurements moving toward the upper-right,
indicating more compact states with increasing
fluctuations. Reproduced with permission
from [44].
D
E is plotted versus the mean of the
distribution
. These values are extracted using
time-resolved FRET. Nanosecond ALEX-based
single-molecule sorting allows the exclusion of
signal from folded CI2 and ACBP. Note that all of
h
E
i
There is one important methodological achievement that should be mentioned
with respect to ALEX-FRET. In [86], a methodology was developed to determine
accurate, unbiased energy transfer ef
ciency E values that proved in many ways to be
more reliable than ensemble FRET. In the nsALEX paper, a second methodology was
developed using single-molecule selection and
fluorescence lifetime
fitting to obtain
accurate E values along with the width of the E distributions down to the nanosecond
time scale. The values obtained by both methods for the same samples matched very
well, even though themanner inwhich the values were obtained were quite different.
In the
first measurements of FRETat the single-molecule level, it seemed that single-
pair FRET would only be useful for measuring dynamic changes in distance. Now,
however, the accuracy and reproducibility of FRET ef
ciency values now rivals, and,
in some cases, surpasses that of ensemble methods (Figure 9.8).
9.4.1.6 Single-pair FRET Studies on Immobilized Proteins
As mentioned earlier, in order to monitor repeated folding and unfolding events it is
necessary to immobilize the proteins. This allows observations over several seconds.
In order to circumvent the dif
culties of surface immobilization, two methods have
been used. First, encapsulation of the proteins in lipid vesicles, and attachment of
those vesicles to surfaces have been used tomonitor such transitions. In this way, the
protein that is folding and unfolding is not held in contact with any surface, and only
comes into contact with the lipid surface of the vesicle, which is relatively inert.
Folding and unfolding were clearly seen, which allowed the timing of these processes