Chemistry Reference
In-Depth Information
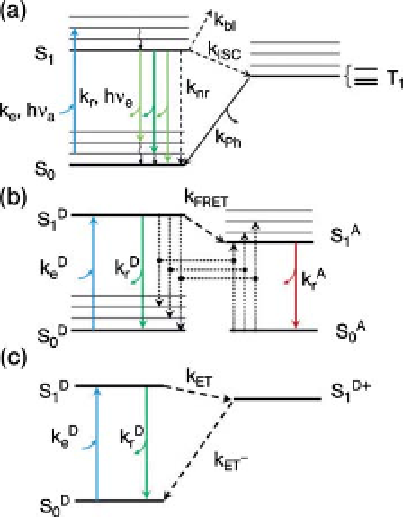
Figure 9.1 Kinetics of fluorescence processes
can be summarized in Jablonski diagrams. (A)
Upon absorption of a photon of energy h
emission (phosphorescence) or non-radiative
relaxation. (B) Fluorescence resonance energy
transfer involves two molecules: a donor D and
an acceptor A whose absorption spectrum
overlaps the emission spectrum of the donor.
Excitation of the acceptor to the lowest singlet
excited state is a process identical to that
described for single-molecule fluorescence (A).
In the presence of a nearby acceptor molecule
(within a few nm), donor fluorescence emission
is largely quenched by energy transfer to the
acceptor by dipole
-
dipole interaction with a rate
k
FRET
R
6
, where R is the D
-
A distance. The
acceptor and donor exhibit fluorescent emission
following the rules outlined in A and omitted in
this diagram for simplicity. (C) Photo-induced
electron transfer effectively oxidizes the donor
molecule with a rate k
ET
n
a
close
to the resonance energy E
S1
-
E
S0
, a molecule in a
vibronic sublevel of the ground singlet state S
0
is
promoted to a vibronic sublevel of the lowest
excited singlet state S
1
. Non-radiative, fast
relaxation brings the molecule down to the
lowest S
1
sublevel in picoseconds. Emission of a
photon of energy h
n
a
(radiative rate k
r
) can
take place within nanoseconds and bring the
molecule back to one of the vibronic sublevels of
the ground state. Alternatively, collisional
quenching may bring the molecule back to its
ground state without photon emission (non-
radiative rate k
nr
). A third type of process present
in organic dye molecules is intersystem crossing
to the first excited triplet state T
1
(rate k
ISC
).
Relaxation from this excited state back to the
ground state is spin-forbidden and thus the
lifetime of this state (1/k
Ph
is in the order of
microseconds tomilliseconds). Relaxation to the
ground state takes place either by photon
n
e
<
h
R), preventing
its radiative relaxation. Upon reduction, the
molecule relaxes non-radiatively to its ground
state. In this scheme, the electron acceptor does
not fluoresce and is therefore not represented.
Reproduced with permission from [8].
exp (
b
In general, the rate k
r
is similar to k
nr
, but k
ISC
,k
Ph
, and k
bl
are orders of magnitude
slower. The lifetime of S
1
is the reciprocal of the sum of rates of all de-excitation
pathways,
t ¼
1
=ð
k
r
þ
k
nr
þ
k
ISC
þ
k
bl
Þ
1
=ð
k
r
þ
k
nr
Þ:
ð
9
:
1
Þ