Chemistry Reference
In-Depth Information
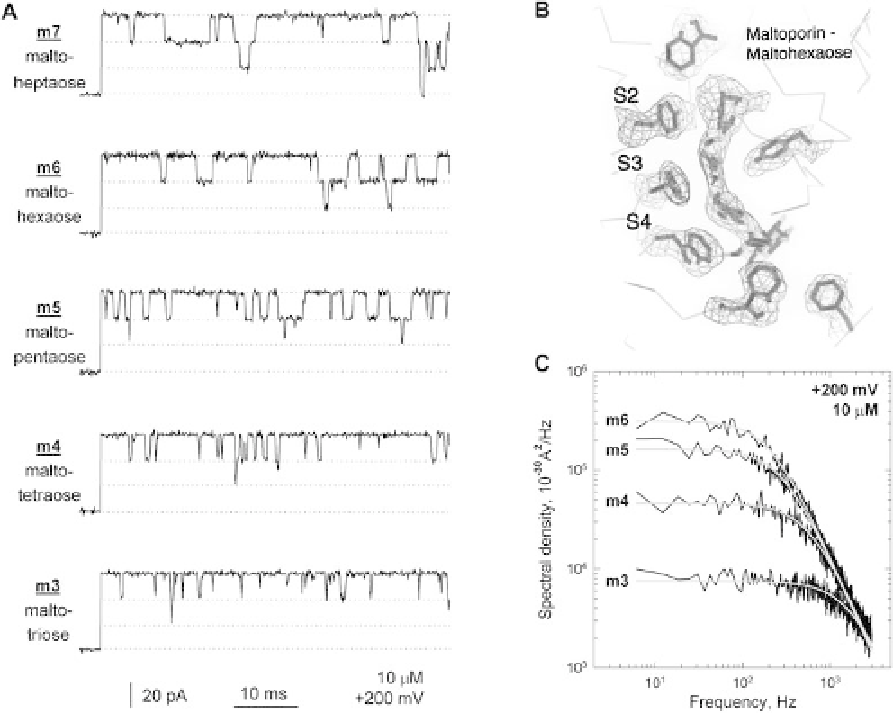
Figure 7.3 (A) Current recordings of single
maltoporin proteins obtained from experiments
where maltodextrins of various lengths were
used. Downward current steps with the
amplitude of one-third of the total current
correspond to time-resolved binding events. The
sugar concentration was 10
chains lining the pore allows the translocation
of the left-handed sugar helix in a screw-like
manner. The three binding subsites S2, S3 and S4
have been shown (taken from [99]).
(C) Power spectral densities of the current
fluctuations induced by reversible binding of
different sugar molecules. The Lorentzian
type spectra indicate a two-state Markovian
process for sugar binding. Spectra were
obtained after subtracting the open channel
noise in the absence of the sugar (taken
from [102]).
M, the applied
transmembrane voltage was 200mV, and the
data were low-pass filtered at 15 kHz (taken
from [102]). (B) Crystal structure of maltoporin
complexed with maltohexose. The helical
arrangement defined by the aromatic lateral
m
such as those occurring in receptor channels. Indeed, a pronounced asymmetry
of channel properties induced by the polarity of the applied voltage has been
demonstrated [88, 92].
Maltoporin serves as the receptor for bacteriophage lambda [95, 107]. Phage
docking to the bacterial surface is followed by injection of the viral DNA. Real-time
formation of the phage
-
receptor complex was recently monitored in planar lipid
bilayers and new hypotheses regarding the binding regions have been postulated
[108, 109].