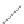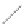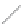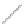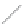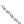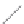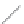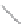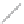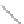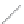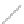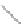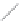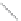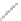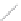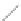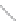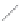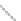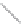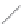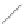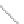Image Processing Reference
In-Depth Information
Si
Si
Si
Si
Si
Si
Si
Si
Si
Si
Si
Si
Si
Si
Si
(a)
(b)
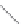
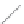
FIGURE 2.2
(a) Schematic diagram of silicon atom; (b) diagram of silicon crystal.
One leftover valence electron is
available for conduction
Si
Si
Si
Si
P
+
Si
Si
P
Si
Si
Si
Si
Si
Si
Si
(a)
(b)
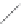
FIGURE 2.3
Schematic diagram of phosphorus-doped silicon crystal: (a) schematic diagram of phosphorus atom; (b) dia-
gram of phosphorus atom in silicon crystal.
One electron is missing: hole
Si
Si
Si
Si
Si
Si
B
−
B
Si
Si
Si
Si
Si
Si
Si
Hole
(a)
(b)
Electron migration
Hole countermigration
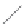
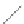
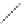
FIGURE 2.4
Schematic diagram of boron-doped silicon: (a) boron atom; (b) boron atom in silicon crystal.
Boron can complete its bonds only by taking an electron from a Si-Si bond, as shown in
Figure 2.4b, leaving behind a hole in the silicon valence band. It is called a hole because it
is a state that an electron should occupy but is missing from and is positively charged due
to the absence of an electron. When another electron moves and fills it in, it can be seen
that the hole moves in the counterdirection, which is regarded as a movement of the hole.