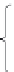Image Processing Reference
In-Depth Information
Fermi level (
E
F
)
Conduction band
Conduction band
Allowed band
Bandgap
Forbidden region
Bandgap
Allowed band
Valence band
Valence band
(a)
(b)
FIGURE 2.1
Schematic energy bands and electron occupancy of allowed band: (a) conductor (metal); (b) semiconductor at
absolute zero and insulator.
The difference between semiconductors and insulators lies in the width of the band-
gap. As the bandgap of semiconductors is small, the density of electrons excited* from the
valence band to the conduction band by thermal energy of 26 meV at room temperature
300 K is substantial.
†
On the contrary, the bandgap of insulators is wide, so the density of
electrons excited to the conductive band is negligible. Thus, electrical conduction is low
and electrical resistance is very high.
In metals, electron density, which contributes to electric current, is very high and there is
no external means to control it. On the contrary, it can be easily controlled in semiconduc-
tors. This is the important source of the function of semiconductor devices.
The material of most real semiconductor LSI circuits is silicon. In silicon devices, elec-
trons that are excited from the valence band to the conductive band, as mentioned above,
are infrequently used. When silicon is used as a starting material for LSI circuits in manu-
facturing, its purity is raised up to 99.999999999% (eleven nines) and a different atom is
added afresh.
‡
However it is added intentionally, it is customarily called an impurity.
As silicon belongs to the IV family of the periodic table, it has four valence electrons,
each of which forms a covalent bond with its nearest neighbor in the silicon crystal, as
shown in Figure 2.2.
As phosphorus (P) and arsenic (As) belong to the V family, they have five-valence elec-
trons. If one silicon atom in the crystal is displaced by one phosphorus or arsenic atom,
four electrons on the phosphorus or arsenic atom form covalent bonds, similar to silicon,
as shown in Figure 2.3. The fifth electron is bound by a positively charged phosphorus or
arsenic ion (P
+
, As
+
) with a binding energy of about 45 meV. However, this electron has a
kinetic energy of 26 meV at room temperature; it leaves the bind easily and moves. Thus,
the fifth electron contributes to conduction. The phosphorus and arsenic atoms are called
donors because they donate an electron to the conduction band by ionization.
The case of applying atoms of the V family has been explained above. But what about
the III family? Boron belongs to the III family and has only three valence electrons. This
means one bonding electron is missing when a silicon atom is replaced by a boron atom in
a silicon crystal, as Figure 2.4 shows.
*
Thermal energy as kinetic energy is converted to potential energy.
†
At absolute zero, semiconductors do not exist but insulators do.
‡
This is called doping, and non-doped silicon is called intrinsic silicon.