Biology Reference
In-Depth Information
To circumvent these problems, we used the heterologous DNA transposon Mos1 to
induce DSBs in the C. elegans genome (
Robert and Bessereau, 2007; Robert et al.,
2008
). Mos1 was initially isolated in D. mauritiana (
Hartl, 2001; Jacobson and Hartl,
1985; Jacobson et al., 1986
) and subsequently mobilized in the C. elegans germ line
(
Bessereau et al., 2001
). Briefly, it was shown that Mos1 copies provided in an
extrachromosomal transgene could insert into the genome when expressing the Mos
transposase in the germ line. It generates a small number of insertions (on average
2.5 insertions per genome) that are stable in the absence of the Mos transposase
(
Williams et al., 2005
). Such insertions are efficiently remobilized in the presence of
the Mos transposase, generating DSBs that are preferentially repaired by homol-
ogous recombination (
Robert et al., 2008
). Based on these observations, we
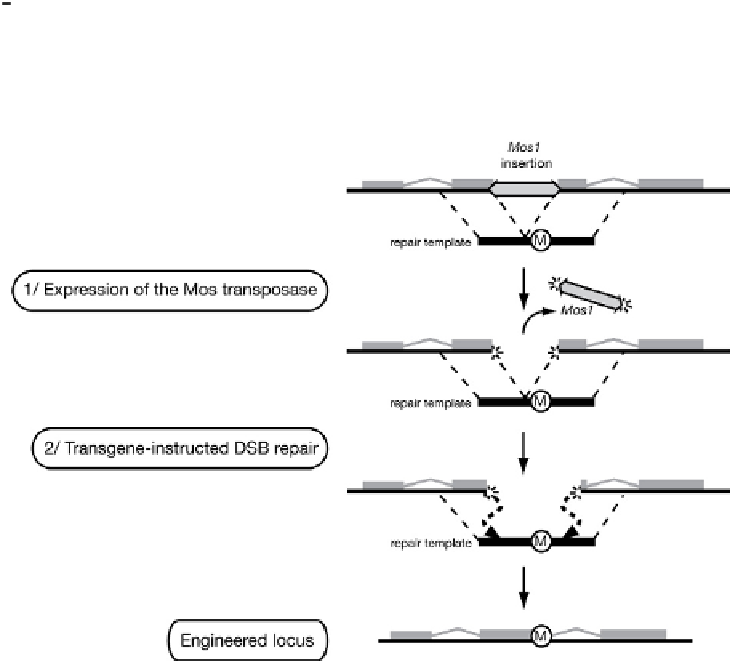
developed a genome engineering technique called MosTIC (for Mos1-excision
transgene-instructed gene conversion;
Fig. 1
)(
Robert and Bessereau, 2007
)and
optimized efficient protocols (
Robert et al., 2009
).
Side-by-side comparison of results obtained with strains carrying repetitive extra-
chromosomal arrays and KI strains generated by MosTIC demonstrates that genomic
Fig. 1
Transgene-instructed gene conversion in C. elegans. A targeted DNA double strand break
(DSB) is created in the genome by triggering the excision of the DNA transposon Mos1 (step 1). A
transgene containing a mutation ''M'' flanked by DNA sequence homologous to the broken locus can be
used as a repair template (step 2). Gene conversion results in the introduction of the mutation in the
chromosome.


Search WWH ::

Custom Search