Biology Reference
In-Depth Information
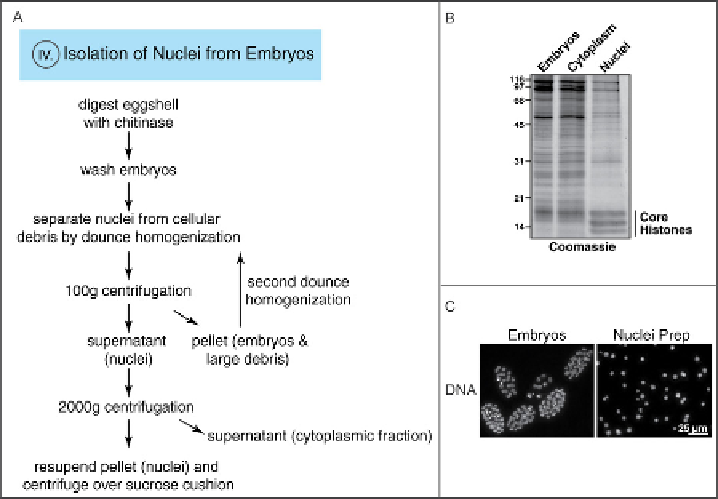
Fig. 6
A) Experimental outline for nuclei isolation. Eggshell of the embryos is digested by chitinase
treatment and embryos are washed. Dounce homogenization in hypotonic buffer separates the nuclei from
cellular debris. Hundred
g centrifugation pellets embryos and cellular debris, which are subject to a
second dounce homogenization. The supernatant containing the nuclei is centrifuged at 2000g to pellet the
nuclei. Nuclei are finally purified by centrifugation over a sucrose cushion. Roman numeral indicates
corresponding section. B) Embryos, cytoplasmic fraction, and isolated nuclei stained with Coomassie.
Histones are enriched in the isolated nuclei. C) Embryo and nuclei preparation stained with Hoechst. (For
color version of this figure, the reader is referred to the web version of this topic.)
4. Spin at 1000g for 3 min.
5. Wash pellet twice with 50 mL cold Embryo Buffer. All subsequent steps are
performed on ice.
6. After final wash, remove supernatant and resuspend embryos in 10 mL 0.5
Nuclei Buffer. Incubate for 15 min on ice to swell cells.
7. Add 10 mL 1
Nuclei Buffer (with 0.1% digitonin and protease inhibitors) and
immediately dounce with
50 strokes in a 15 mLWheaton dounce homogenizer
with a B pestle (tight fit).
8. Spin at 100g for 3 min. This centrifugation pellets large debris.
9. Remove supernatant that contains the nuclei and keep on ice.
10. Resuspend pellet in 10 mL Nuclei Buffer (with 0.1 % digitonin and protease
inhibitors) and dounce pellet as described in step 7.
11. Spin at 100g for 3 min and combine two supernatants.
12. Spin at 2000g for 15 min to pellet nuclei. Supernatant is cytoplasmic fraction.
Search WWH ::

Custom Search