Environmental Engineering Reference
In-Depth Information
IdoUA unit by the C
5
-Epimerase. After further chemical persulfation by pyridine
sulfate and finally selective desulfation, a heparin mimic, known as neoparin, was
generated. Although neoparin has levels of anti-Xa and anti-IIa activity similar
to those of heparin, unwanted products, such as 3-
O
-sulfo GlcUA/IdoUA, were
present in the polysaccharide, suggesting a limitation in the selectivity of chemical
sulfation/desulfation in HS synthesis.
4.3 Enzymatic Redesign of HS
Our lab, in collaboration with the Linhardt group, has also developed an enzymatic
approach to the synthesis of bioactive HS polysaccharides from HS backbone using
the same strategy, starting from the chemically desulfated
N
-sulfated (CDSNS) hep-
arin [40]. Only three enzymatic steps are required to synthesize anticoagulant HS
(HS1 in Fig. 6) in milligrams. Immobilized enzymes were employed to permit reuse
and to improve the stability of HS sulfotransferases. We further tested the activity of
HS1 in inhibiting factor Xa and IIa. As expected, HS1 is a potent inhibitor of factor
Xa and IIa via AT-mediated process. Its inhibition activity is very similar to that of
OH
OH
HOOC
O
O
O
O
O
O
O
HO
HO
O
CDSNS Heparin
HO
HO
NHSO
3
-
HOOC
O
OH
OH
NHSO
3
-
2OST
OH
OH
HOOC
O
O
O
O
O
O
O
HO
HO
O
HO
HO
NHSO
3
-
O
HOOC
OH
O
SO
3
-
NHSO
3
-
6OST
O
SO
3
-
O SO
3
-
HOOC
O
O
O
O
O
O
O
HO
HO
O
HO
HO
NHSO
3
-
HOOC
O
OH
OSO
3
-
NHSO
3
-
3OST1
OSO
3
-
OSO
3
-
HOOC
O
O
O
O
O
O
O
HS1
HO
O
HO
HO
NHSO
3
-
-
O
3
S
O
OH
HOOC
O
OSO
3
-
NHSO
3
-
Fig. 6
Enzymatic synthesis of anticoagulant CDSNS heparin. The synthesis was begun with
chemical de-sulfated/
N
-sulfated (CDSNS) heparin. CDSNS heparin was modified by 2-OST,
6-OST and 3-OST1 to generate anticoagulant polysaccharide HS1



























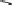






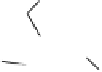
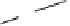



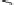















Search WWH ::

Custom Search