Environmental Engineering Reference
In-Depth Information
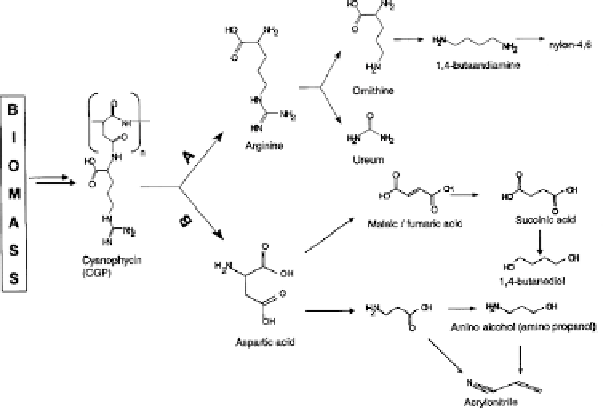
Fig. 4
Potential reaction products of cyanophycin from arginine (route A) and aspartic acid
(route B)
already been described that the formation of ornithine and urea may be obtained
using arginase enzyme, however hydrolysis of arginine may also be achieved by
chemical hydrolysis [40-42]. The use of the enzyme offers the advantage of the
use of ambient temperatures and no further decomposition of urea. However, issues
of improving conversion rates and operational stability form an important role for
successful large scale application. Alternatively, the use of chemical hydrolysis
may be interesting due to potentially lower contact times and costs of reagents,
however conditions can lead to an array of products (via cyclisation for example)
and degradation of urea [40-42] which leads to losses in conversion and makes
further downstream processing more complex (and costly).
In pathway B, the conversion of aspartic acid by
-deamination to fumaric acid
has been described and leads to the formation of commercially interesting prod-
ucts such as fumaric acid, which in turn could be used as a precursor for other
monomers for polymeric materials, such as 1,4-butanediol for poly(butylene tereph-
thalate). Since this route results in the loss of ammonia, it is considered that the
optimum use of the functionality is not achieved, for reasons that we described
earlier. Thus research has focused on the conversion of aspartic acid to
α
β
-alanine.
α
-Decarboxylation of aspartic is not trivial. Only limited reports of (photo)chemical
transformation are available [43]. The alternative to carry out this transformation is
to use the enzyme, aspartic acid alpha-decarboxylase. This enzyme has not been
widely studied with respects to its immobilisation, activity, stability and re-use
although this forms the basis to determine the potential for efficient use at large scale
for the synthesis of commercial products. A manuscript is has recently been submit-
ted which describes these issues in more detail [44]. The conversion of
-alanine to
a range of chemicals, including those given in Fig. 4 are now being studied.
β
Search WWH ::

Custom Search