Graphics Programs Reference
In-Depth Information
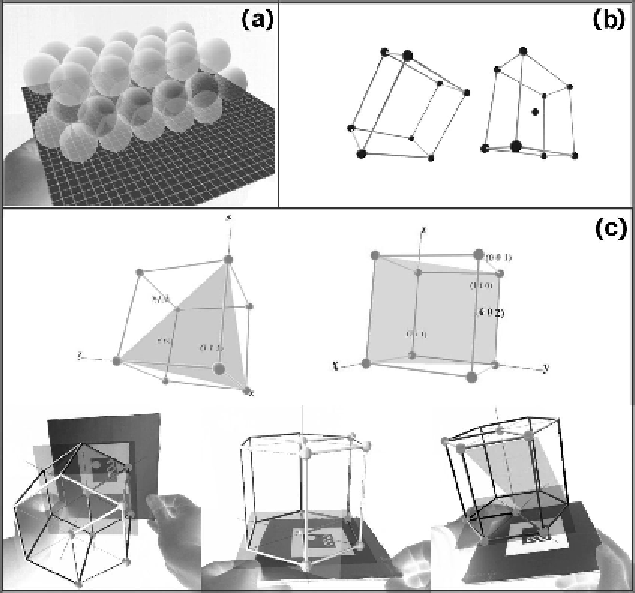
Figure 6. Some models developed to understand the basic elements of crystalline materials: (a) Hex-
agonal Close-Packed Crystal Structure; (b) Bravais lattices: Tetragonal P and Tetragonal I; and (c)
models to understand crystallographic planes and Miller indices
according to different geometries. There are seven
crystallographic unit cells of crystals, according
to their axes of symmetry. For example, there are
the cubic system with four threefold axes, and the
triclinic system with no symmetry axes. 14 Bravais
lattices can be defined that can be primitive cells
(P), cells with lattice points in the center (I), or
cells with lattice points in the center of the faces
(F). Figure 6b shows some of them.
The crystallographic planes are designated by
the Miller indices (hkl), which must be determined
from reciprocals of fractional axial intercepts. Any
two planes parallel to each other are equivalent
and have identical indices. For crystals having
hexagonal symmetry, there is a convention in the
use of four indices, the Bravais-Miller indices
(hkil). These all represent complicated problems
that the students must solve obtaining the crystal-
lographic planes or the Miller indices for a plane
that is drawn within a unit cell.
Because of this, we have modeled two struc-
tures, a cubic and a hexagonal structure both with
some planes inside. Students have to find and
designate these planes (see Figure 6c). In order to
help the students, planes may appear or disappear
as they move the structures. This occurs because
the models are designed so that one plane does
not interfere with the other planes.
To understand crystal structures, we have
modeled the most important ones, trying to under-
line their main aspects. For example, the spinel,
MgAl
2
O
4
, can be described as an FCC lattice of
O
2-
ions while Mg
2+
ions fill the tetrahedral sites
and Al
3+
the octahedral ones (see Figure 7a).
Search WWH ::

Custom Search