Environmental Engineering Reference
In-Depth Information
50
40
30
20
10
0
470
490
510
530
T
(K)
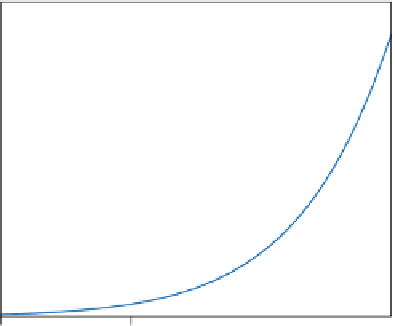
FIGURE 17.2
The FT reaction rate according to the Yates and Satterfield expression as a
function of temperature.
The Yates and Satterfield expression just describes the conversion of syngas by FTS; it
does not say anything about the structure of the obtained products. The most important
products are linear alkanes (
n
-paraffins, hydrocarbons with only single C
-
C bonds),
but also considerable amounts of alkenes (olefins, hydrocarbons with a C
C bond)
and branched alkanes can be produced. In addition, small amounts of oxygenated hydro-
carbons such as alcohols are produced. FTS can be considered as a polymerization process
taking place at the surface of a catalyst. By adding more carbon atoms, the chains become
longer and longer (propagation), until the chain growth is terminated (see Figure 17.3a). In
every step, the probability for propagation is
═
α
, while the probability for termination is
(1
—
determines the product distribution (see Figure 17.3b). This distribution is named the
Anderson
−
α
). This means that the parameter
α
—
denoted as the chain growth probability
Flory distribution, after the proposers of this mechanism. In most
cases, the aim is to produce liquid hydrocarbons, i.e., molecules with a considerable num-
ber of carbon atoms. This means that
-
Schulz
-
α
should be high enough; typical values in practice
are around 0.9. The fact that at high
also heavywaxes (
n
>20) areproduced is seenas less
of a problem: these can be used invarious applications (e.g., as lubricant and for coating) or
can be easily cracked to shorter molecules. Ways to increase
α
are to operate at lower H
2
:
CO ratio or at lower temperature. The latter has its limitations, since it will also reduce the
reaction rate.
α
17.2.2 Catalyst Aspects
The FTS requires a solid-phase catalyst to enhance the reaction rate. The two main
catalyst types applied are cobalt and iron. Most current processes use cobalt: it is
more active (can be used at a lower temperature) and less prone to sintering, but it
















Search WWH ::

Custom Search