Environmental Engineering Reference
In-Depth Information
Current low
End plate
Anode
Electrolyte
Cathode
Repeating unit
Oxidant low
Anode
Bipolar
separator
Fuel low
FIGURE 16.2
A typical planar SOFC configuration. (Source: Reproduced with permission
from Stambouli and Traversa (2002). © Elsevier Science Ltd.)
can be used as cathode materials for SOFCs because of the high operating temperature.
Noble metals are not desirable for practical applications because of their prohibitive
cost and insufficient long-term mechanical stability. Perovskite-type lanthanum
strontium manganite (LSM) provides excellent thermal expansion matching with
zirconia electrolytes and performs well at operating temperatures above 800
C.
For operation at lower temperatures, mixed ionic/electronic conducting ceramics,
such as the perovskite lanthanum strontium cobalt ferrite (LSCF), are under serious
consideration. Currently, several new cathode materials are under development for
SOFCs, but a detailed description of such materials is beyond the scope of this topic.
The reader is referred to the overview works of Skinner (2001) and Jacobson (2010).
16.2.5 Stack Design
To reach sufficient power levels, FC are interconnected to form stacks. There are dif-
ferent stack design concepts based on tubular and flat-plate cells. Figure 16.2 shows a
planar SOFC configuration. Interconnects, which are made of ceramic or metallic com-
pounds depending on the operating temperature, connect the cells within the stack.
In comparison to planar designs, tubular designs do not require a specific seal to
isolate the oxidant from the fuel, which makes the performance of the tubular cell
highly stable over long-term operation. A schematic view is presented in Figure 16.3.
16.3 BIOMASS GASIFIER-SOFC COMBINATION
Considering biosyngas as a fuel for use in SOFCs raises many critical issues. One sig-
nificant task is to define the tolerance limits of the anode for contaminants such as tar,




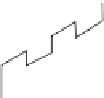



























Search WWH ::

Custom Search