Environmental Engineering Reference
In-Depth Information
contribute to the substrate alkalinity. This is due to the fact that upon degradation of
a neutralized organic acid, the cation responsible for neutralization receives a bicar-
bonate molecule as counterion, herewith contributing to the pH buffer capacity of the
system. Also, other forms of alkalinity may play an important role in the anaerobic
digestion process, e.g., phosphates or precipitates of cations like calcium or
magnesium that upon solubilization at decreasing pH values will contribute to the
pH buffer capacity of the system. Figure 14.3 (left) shows an overview of the main
pH-determining reactions occurring in the anaerobic digestion process.
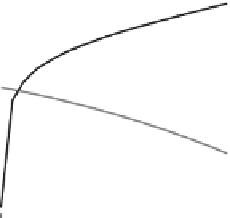
Figure 14.3 (right) shows a graph of the pH and biogas composition upon degra-
dation of 50 mM glucose to biogas as a function of the alkalinity in the system. The
graph shows that at increasing alkalinity the pH increases. This is due to two reasons:
first, the alkalinity results in elevated bicarbonate concentrations in the system, and
second, due to bicarbonate formation, the carbon dioxide partial pressure in the biogas
decreases. In the bicarbonate
carbon dioxide pH buffering system, bicarbonate repre-
sents the base and carbon dioxide is the acid, and more base and less acid evidently
mean an increasing pH. It is furthermore evident that at high alkalinity values (> ~50
mM), the pH in the anaerobic digestion process is strongly buffered, and pH instabil-
ities due to instabilities in biological acid or base production will not result in strong
variations in the pH. A limitation of strong pH buffering is that problems with the
process performance can hardly be observed from changes in the pH at high alkalinity
concentrations.
To predict the biogas composition from substrate characteristics, the elemental
composition needs to be determined. However, this is not a standard measurement
for heterogeneous substrates typically used for the anaerobic digestion process. The
elemental substrate composition can be estimated from three standard measurements
-
8.0
7.5
7.0
6.5
6.0
5.5
5.0
4.5
4.0
0.9
CH
4
(g) CO
2
(g)
k
L
a
CH
4
,
K
h
, CH
4
0.8
pH
k
L
a
CO
2
,
K
h
, CO
2
0.7
CH
4
0.6
Substrate,
C
c
H
h
O
o
N
n
L
CH
4
+ CO
2
0.5
K
a
,H
2
CO
3
0.4
CO
2
Alkalinity,
NH
+1
, Na
+1
,Ca
+2
HCO
-1
+ H
+1
0.3
0.2
K
a
,HCO
-1
0.1
CO
3
-2
+ H
+1
0
0
0.05
0.1
Alkalinity (eq.L
-1
)
FIGURE 14.3
Schematic overview of the relation between substrate composition, alkalinity,
biogas composition, and pH in the anaerobic digestion process.
k
L
a
is the volumetric gas
liquid
mass transfer coefficient, whereas
K
h
and
K
a
are thermodynamic equilibrium constants for
gas-liquid partitioning and acid-base equilibrium, respectively. The graph on the right
shows the pH and biogas composition upon degradation of 50 mM glucose to methane and
inorganic carbon as a function of the alkalinity concentration in the system.
-













































Search WWH ::

Custom Search