Environmental Engineering Reference
In-Depth Information
100
Hemicellulose
Cellulose
Lignin
Wood
80
60
40
20
0
100
200
300
Temperature (°C)
400
500
FIGURE 11.3
TGA of wood, cellulose, hemicellulose and lignin in nitrogen. A typical result
is shown at ca. 10 Kmin
-1
. Data taken from Basu (2010).
Hydroxyacetaldehyde
Acetol
Ethylene glycol
……
Fragmentation
K
+
Ca
2+
Na
+
…
catalyzed
Depolymerization
Active cellulose
(lower DP)
Cellulose
Crosslinking
K
+
Ca
2+
Na
+
…
catalyzed
Depolymerization
Levoglucosan
Cellobiosan
Larger oligomer anhydrosugars
Char, gas, water
FIGURE 11.4
Cellulose depolymerization (modified Waterloo model). From Piskorz
et al. (1989).
An often used reaction path scheme for cellulose pyrolysis is shown in Figure 11.4.
In this scheme, solid cellulose decomposes firstly into an (liquid) intermediate, often
termed active cellulose, with a lower DP. The active cellulose can depolymerize fur-
ther into anhydrosugars or react via fragmentation (e.g., ring opening) or cross-linking
reactions, which are catalyzed by alkali metals such a potassium, sodium, and mag-
nesium (present in the ash).
For the pyrolysis of pure cellulose, the depolymerization reaction is rather selective
with levoglucosan yields of up to 70% reported. However, it is known that while
pyrolysis of cellulose can result in such high levoglucosan yields, a much smaller part
of the cellulose fraction of lignocellulosic materials can be currently converted into
sugars in fast pyrolysis reactors. Overall, it can be concluded that detailed chemical
knowledge is available for pure cellulose, whereas for hemicelluloses, lignin, and real
lignocellulosic biomass, this understanding is still in its early stages.





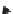













Search WWH ::

Custom Search