Environmental Engineering Reference
In-Depth Information
introduction to the subject; more general information on fast pyrolysis can be found in
Basu (2010), Bridgwater (2008, 2012), Kersten and Garcia-Perez (2013), Mohan et al.
(2006), and Venderbosch and Prins (2010).
11.2 A FIRST LOOK AT A LIQUEFACTION PROCESS
At the level of the mass balance, any liquefaction process can be described by
Biomass
!
solid residues + vapors organics + H
2
O
f
g
+ permanent gases
ð
RX
:
11
:
1
Þ
Solid residues are carbonaceous and contain a large amount of the ash-containing
compounds present in the feedstock. Permanent gases are typically CO
2
, CO, CH
4
,
and H
2
. Water can already be present in the feed and is also produced in the reactions.
Organics are multicomponent mixtures containing mostly oxygenated hydrocarbons.
In the pyrolysis reactor, these organics are present as vapors and aerosols, while after
condensation they are collected together with the water as a liquid, which can contain
particulate fragments. During hydrothermal liquefaction and solvolysis, the vast
majority of the organics remain in the liquid phase. Core elements in a liquefaction
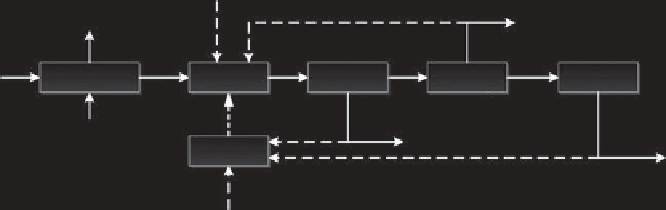
plant are (i) pretreatment (drying, cutting, etc.), (ii) reactor, (iii) heat production sys-
tem, (iv) heat transfer to the reactor, (v) liquid recovery and collection, (vi) solid res-
idue removal and utilization, and (vii) gas recovery and utilization. This archetype
process is visualized in Figure 11.1.
Many of the proposed processes utilize the produced char and/or gases to generate
the energy required for the process. In this way, no additional energy is necessary.
In some hydrothermal/solvolysis processes, the liquid reactor effluent is recycled.
The yield of the liquid organic product typically varies between 40 and 65 wt%.
Yield is defined here as kg organic liquid produced per kg of the organic part of
the biomass fed (i.e., dry ash free). Hereafter, all yields reported in this chapter are
on dry ash free basis.
Additional chemicals/catalyst
Liquid (oil)
e.g. H
2
O, minerals
Solid
recovery
Liquid
recovery
Gas
recovery
Biomass
Pretreatment
Reactor
Heat transfer
e.g. Heat, electricity
Char + ash
Heat
generation
Non-condensable
vapors (gas)
Other fuel
FIGURE 11.1
Archetype conceptual liquefaction process.








Search WWH ::

Custom Search