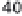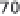Environmental Engineering Reference
In-Depth Information
No other compounds are now synthesized in such
quantities as sulfuric acid and ammonia. H
2
SO
4
has been
the most important industrial chemical since the begin-
ning of modern syntheses during the nineteenth century.
In contrast, ammonia synthesis from its elements became
possible only with the invention of a catalytic high-
pressure process by Fritz Haber in 1909 and its rapid
commercialization over the next four years by BASF, the
German chemical company, under the leadership of Carl
Bosch (Smil 2001). Global production was limited until
1950, nearly doubled during the 1950s, further qua-
drupled by 1975, and after a brief period of stagnation
in the late 1980s, reached about 120 Mt by 2000. Dur-
ing that year, H
2
SO
4
synthesis amounted to 157 Mt, but
because of ammonia's lower molecular weight (17 vs. 98),
ammonia is the world's leading chemical in terms of syn-
thesized moles (nearly five times as much as H
2
SO
4
).
The energy requirements of chemical syntheses com-
prise the heat equivalents of fuels and electricity used in
synthesis and the energy embodied in the feedstocks.
Ammonia's lower heating value is 18.6 GJ/t, and the
stoichiometric energy requirement for ammonia synthesis
is 20.9 GJ/t. After taking credit for the purge gas (drawn
in order to control the concentration of inert gases in the
recycled flow), net feedstock inputs of modern ammonia
plants are very close to this value. The reduction of en-
ergy needed for NH
3
synthesis has been impressive (Hel-
sel 1987; Kongshaug 1998; Smil 2001). Coke-based
Haber-Bosch synthesis in the first commercial plant in
Oppau, Germany, needed initially more than 100 GJ/t
NH
3
, and typical pre-WW II plants required around 85
GJ/t NH
3
(fig. 10.6).
During the 1950s natural gas-based synthesis with
low-pressure reforming (0.5-1 MPa) and with recipro-
cating compressors needed 50-55 GJ/t NH
3
. In the
10.6
Declining specific energy cost of Haber-Bosch ammonia
synthesis, 1913-2000. From Smil (2001).
early 1960s total energy consumption in plants working
with reformer pressure of 1 MPa and synthesis loop pres-
sure of 35 MPa would have added up to 46-50 GJ/t
NH
3
. A decade later the best large single-train plants,
with high-pressure reforming (
>
3 MPa) and with centrif-
ugal compressors producing converter pressure 15 MPa,
reduced the energy need to 35 GJ/t NH
3
. Reciprocating
compressors powered by electric motors needed 520-
700 kWh/t NH
3
, and steam turbine-driven centrifugal
compressors in much larger post-1963 plants consumed
as little as 20-35 kWh/t NH
3
.
By the early 1980s further gradual efficiency improve-
ments had brought the average energy needs of ammonia