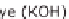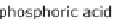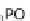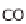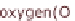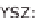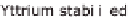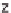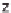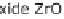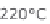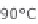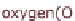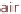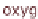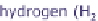Environmental Engineering Reference
In-Depth Information
■
AFC alkaline fuel cell
■
PEFC polymer electrolyte fuel cell
■
PEMFC proton exchange membrane fuel cell
■
DMFC direct methanol fuel cell
■
PAFC phosphoric acid fuel cell
■
MCFC molten carbonate fuel cell
■
SOFC solid oxide fuel cell
Figure 13.6 shows the respective fuel gases and oxidation materials as well as the
electrolytes and operating temperature ranges for the different types of fuel cells.
Figure 13.6
Differences between fuel cell types.
The proton exchange membrane fuel cell (PEMFC) is the one most frequently used
today. In this fuel cell the electrolyte consists of a proton-conductive polymer fi lm.
The fuel gases fl ow through carbon or metal substrates which serve as electrodes.
The substrates have a platinum coating that acts as the catalyzer. The typical operat-
ing temperature is about 80 °C. These cells do not require pure oxygen for operation
but can also work with normal air.
Because hydrogen as an energy source is only available in limited quantities today,
there is an interest in using fuel cells directly with energy sources like natural gas
and methanol that are relatively easily available. At a preliminary stage a reformer
uses a chemical process to break down hydrocarbons such as natural gas into hydro-
gen and other components. In the process a hydrogen-rich reformat gas is formed
from which a gas purifi cation stage is still needed to eliminate harmful carbon
monoxide (CO) for the fuel cell.