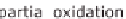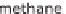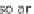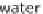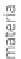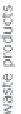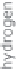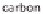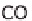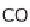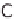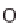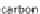Environmental Engineering Reference
In-Depth Information
13.1.1 Production of Hydrogen
Hydrogen must fi rst be produced in a pure form before the energy from it can be
used (Figure 13.2). This requires an easily available inexpensive raw material con-
taining hydrogen. Aside from water (H
2
O), which consists of hydrogen (H) and
oxygen (O), hydrocarbon compounds can also be an option. This is primarily natural
gas, or methane (CH
4
). Heating oil and coal consist of hydrogen (H) and carbon (C)
but have a much higher proportion of carbon than natural gas.
Figure 13.2
Procedure for producing hydrogen.
Current industrial methods for producing hydrogen almost exclusively use fossil
fuels, such as natural gas, crude oil or coal, as the raw material. Methods such as
steam reforming or partial oxidation to produce hydrogen from fossil hydrocarbons
chemically separate the carbon. It then reacts to carbon monoxide (CO), which can
be used energetically. The end product is carbon dioxide (CO
2
). These methods for
producing hydrogen are not real options for actively protecting the climate.
The Kværner method also uses hydrocarbons as the base material. However, the
waste product that it produces is active coal - therefore, a pure carbon. A direct
formation of carbon dioxide can be prevented with this method if the carbon is not
burnt further.
Basically, all the methods mentioned to produce hydrogen from fossil energy
sources are run at high processing temperatures. This requires large amounts of
energy. If this energy comes from fossil sources, this will again lead to the emission
of carbon dioxide. For climate protection it is usually better to burn natural gas or
oil directly than to take the circuitous route of producing hydrogen and then using
it as a supposedly environmentally friendly fuel.