Geoscience Reference
In-Depth Information
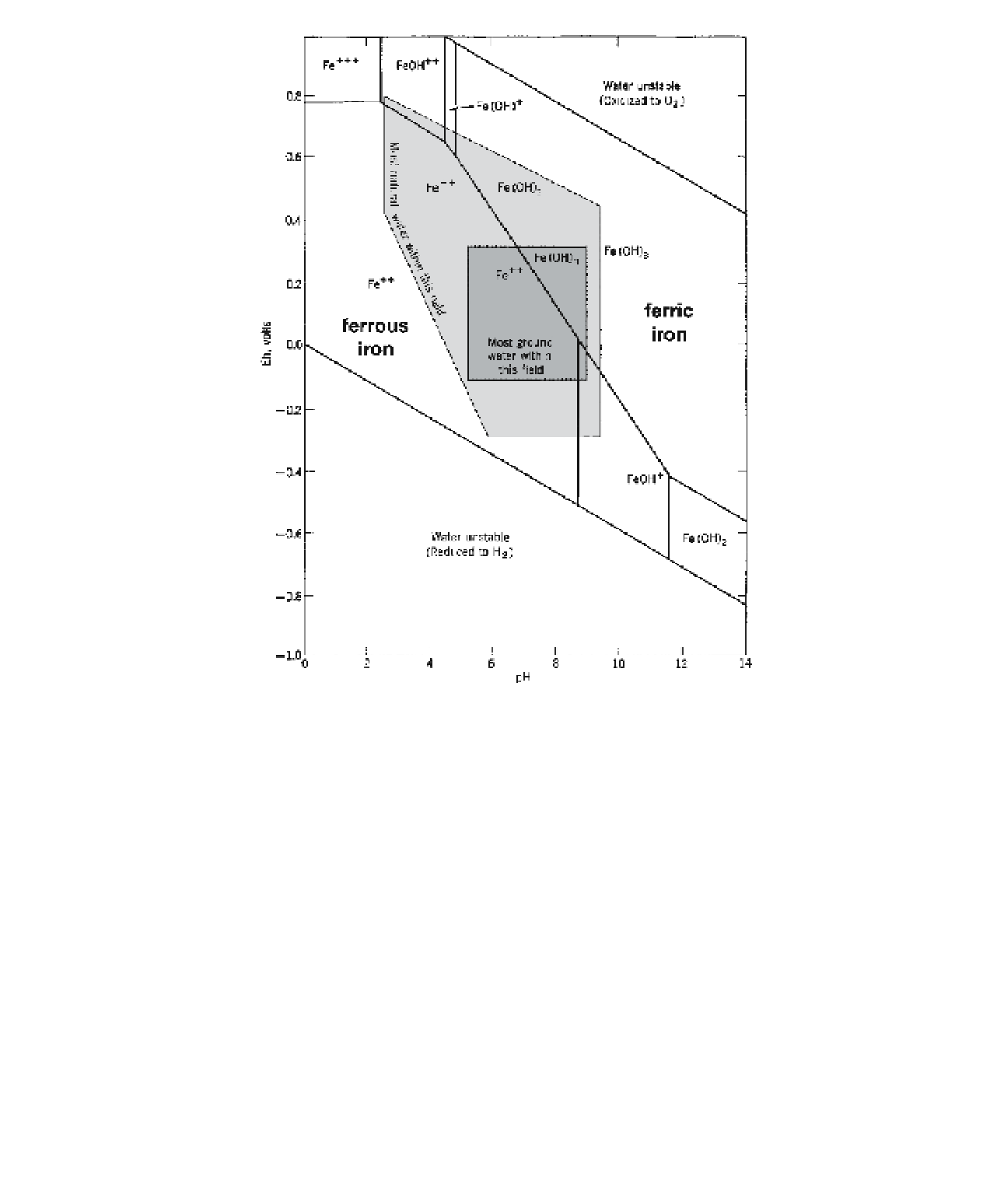
Figure 4-22.
Eh-pH diagram for iron. The main boundary between ferrous and ferric iron runs diagonally across the
middle of the i eld in which most natural waters occur. Slight changes in either Eh or pH may affect the valence state of
iron. Adapted from Davis, S.N. and DeWiest, R.J.M. 1966.
Hydrogeology
. John Wiley & Sons, New York (Fig. 3.11).
waters contain dissolved solids, soluble organic
compounds, and dissolved gases in solutions
ranging from highly acidic to highly alkaline
and from well oxygenated to lacking in free
oxygen. In wetland environments, acidity (pH)
and oxidation potential (Eh) are two key factors
that control many chemical reactions that may
take place.
Figure 4-23.
Iron-cemented rødsten (redstone) formed
where ground water emerged from a gravel bed
exposed in the cliff (see Color Plate 4-23). Photo by
J.S. Aber; Ristinge Klint, Denmark.