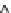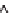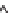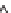Agriculture Reference
In-Depth Information
Algal and plant growth in such lakes tends to be strongly limited by P availability
(Wetzel 2001, Schindler 2012). Above a pH of ~9, the co-precipitation of phos-
phate with calcite contributes to the limited availability of P. Sedimentation of this
co-precipitated P serves as a negative feedback mechanism for aquatic primary
production, potentially ameliorating P-driven eutrophication (Koschel et al. 1983,
Hamilton et al. 2009). Calcite precipitation can also act as a negative feedback to
aquatic primary production by attenuating light in the water column and by floc-
culation and consequent sedimentation of algal cells (Koschel et al. 1983). Calcite
precipitation and deposition on underwater surfaces impact the ecology and bio-
geochemistry of lakes in many other ways, for example, by binding trace met-
als and dissolved organic matter and by smothering biofilms and underwater plant
leaves (Kelts and Hsü 1978, Wetzel 2001).
Figure 11.12
. Concentrations of total dissolved phosphorus (P) and nitrate (NO
3
-
) in lakes
(A, B) and wetlands (C, D), in relation to the importance of groundwater as indicated by
magnesium (Mg
2+
) concentrations. Groundwater at equilibrium with dolomite tends to con-
tain ~2 meq L
-1
of Mg
2+
whereas the concentration of Mg
2+
in precipitation is negligible (Fig.
11.11). Data based on 152 lakes across southern Michigan, most sampled once in the sum-
mer, and 17 wetland sites in the KBS area, many sampled multiple years in May and Oct;
sampling was conducted from 1996-2008.