Agriculture Reference
In-Depth Information
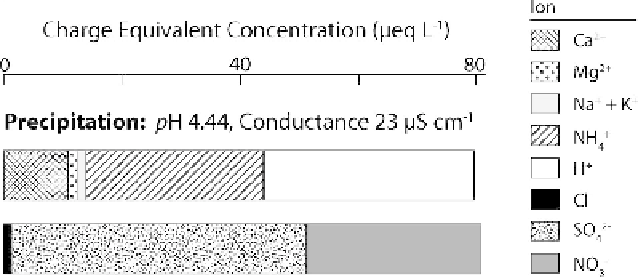
Figure 11.5
. Ionic composition of precipitation at KBS, expressed as charge equivalents.
Data are volume-weighted means for the 30-year monitoring record (1979-2008; see Table
11.1 for sources).
has increased from a mean of 4.34 in the 1980s to 4.83 from 2006 to 2010, and total
acid deposition has fallen from 0.44 to 0.14 kg H
+
ha
−1
yr
−1
in the same time frame.
Similar trends have been observed throughout the midwestern and eastern United
States and likely reflect reduced emissions of S and N oxides due to tighter environ-
mental controls on anthropogenic sources (Driscoll et al. 2001, Kahl et al. 2004).
Natural Chemical Changes as Water Moves through Soils
Water from precipitation changes greatly in chemical composition as it percolates
through soils. Some of these changes reflect dissolution of materials at or near the
soil surface, but as water percolates through the upper few meters of the soil profile,
mineral weathering changes its chemistry further. Because the soils and underlying
glacial deposits in the vicinity of KBS are recently formed, they still contain an
abundance of readily weathered minerals. Groundwater at KBS begins to acquire
its chemical signature within a few meters of the soil surface.
Upland soils in the KBS landscape are generally well-drained loams that devel-
oped under deciduous forest (Robertson and Hamilton 2015, Chapter 1 in this vol-
ume). The forest was interspersed with smaller areas of oak openings, prairie, and
savanna before most areas were converted to agriculture during European settle-
ment in the mid-1800s (Chapman and Brewer 2008). In recent decades, row-crop
agriculture has continued on the relatively level outwash plains, while secondary
forest has developed on much of the less productive land that was abandoned from
agriculture during the 1900s. The most common soil formations are alfisols that
cover most of the upland areas, with histosols, mollisols, and entisols occupying
lower-lying areas around lakes, wetlands, and streams (Schaetzl 2009). Soil miner-
alogy on upland soils at KBS is dominated by quartz, K-feldspar, plagioclase, and
amphibole (Jin et al. 2008a).
Carbonate minerals (calcite and dolomite) are abundant in the glacial deposits
from which KBS soils are derived. Acid precipitation has little effect on most sur-
face water bodies in the region because the acidity is neutralized as water passes