Environmental Engineering Reference
In-Depth Information
quality chemical energy stored in all the oil deposits of
Saudi Arabia. Yet because the ocean's heat is so widely
dispersed, it cannot be used to move things or to heat
things to high temperatures.
15
The First Law of Thermodynamics
In a physical or chemical change, we can change
energy from one form to another but we can never
create or destroy any of the energy involved.
Scientists have observed energy being changed from
one form to another in millions of physical and chemi-
cal changes. But they have never been able to detect
the creation or destruction of any energy (except in nu-
clear changes). The results of their experiments have
been summarized in the
law of conservation of en-
ergy,
also known as the
first law of thermodynamics:
In all physical and chemical changes, energy is neither cre-
ated nor destroyed, although it may be converted from one
form to another.
This scientific law tells us that when one form of
energy is converted to another form in any physical or
chemical change,
energy input always equals energy out-
put.
No matter how hard we try or how clever we are,
we cannot get more energy out of a system than we put
in; in other words,
we cannot get something for nothing in
terms of energy quantity.
This is one of Mother Nature's
basic rules.
10
5
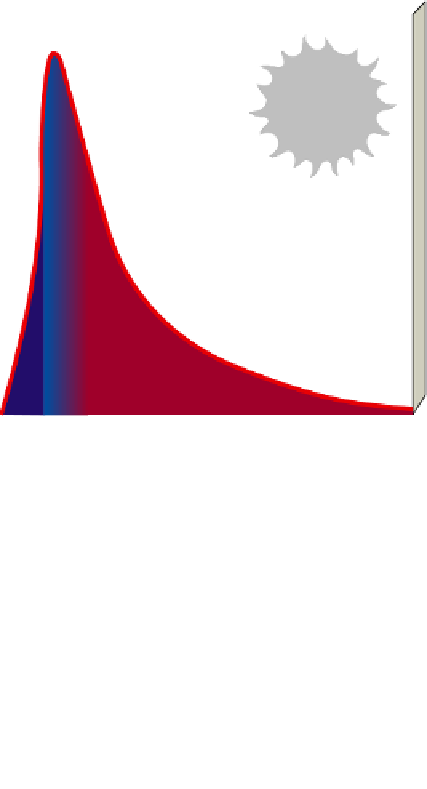
Visible
Infrared
0
0.25
1
2
2.5
3
Wavelength (micrometers)
Active Figure 2-9
Solar capital:
the spectrum of electromag-
netic radiation released by the sun consists mostly of visible
light.
See an animation based on this figure and take a short
quiz on the concept.
When a car engine burns gasoline, the potential energy
stored in the chemical bonds of its molecules changes
into heat, light, and mechanical (kinetic) energy that
propel the car. Potential energy stored in a flashlight's
batteries becomes kinetic energy in the form of light
when the flashlight is turned on. When your body uses
potential energy stored in various molecules to do
work, it becomes kinetic energy.
The Second Law of Thermodynamics
Whenever energy changes from one form to another,
we always end up with less usable energy than we
had initially.
Because the first law of thermodynamics states that
energy can be neither created nor destroyed, you may
be tempted to think there will always be enough en-
ergy. Yet if you fill a car's tank with gasoline and drive
around or use a flashlight battery until it is dead,
something has been lost. But what is it? The answer is
energy quality,
the amount of energy available that can
perform useful work.
Countless experiments have shown that when en-
ergy changes from one form to another, a decrease in
energy quality or ability to do useful work always oc-
curs. The results of these experiments have been sum-
marized in the
second law of thermodynamics:
When
energy changes from one form to another, some of the useful
energy is always degraded to lower-quality, more dispersed,
less useful energy.
This degraded energy usually takes
the form of heat given off at a low temperature to the
surroundings (environment). There it is dispersed by
the random motion of air or water molecules and be-
comes even less useful as a resource.
In other words,
we cannot even break even in terms of
energy quality because energy always goes from a more use-
ful to a less useful form when it changes from one form to
Witness how kinetic and potential energy might be used by
a Martian at Environmental ScienceNow.
Energy Quality
Energy can be classified as having high or low
quality depending on how useful it is to us as a
resource.
Energy quality
is a measure of an energy source's abil-
ity to do useful work, as described in Figure 2-10.
High-quality energy
is concentrated and can per-
form much useful work. Examples include electricity,
the chemical energy stored in coal and gasoline, con-
centrated sunlight, and the nuclei of uranium-235,
used as fuel in nuclear power plants.
By contrast,
low-quality energy
is dispersed and
has little ability to do useful work. An example is heat
dispersed in the moving molecules of a large amount
of matter (such as the atmosphere or a large body of
water) so that its temperature is low.
For example, the total amount of heat stored in the
Atlantic Ocean is greater than the amount of high-