Environmental Engineering Reference
In-Depth Information
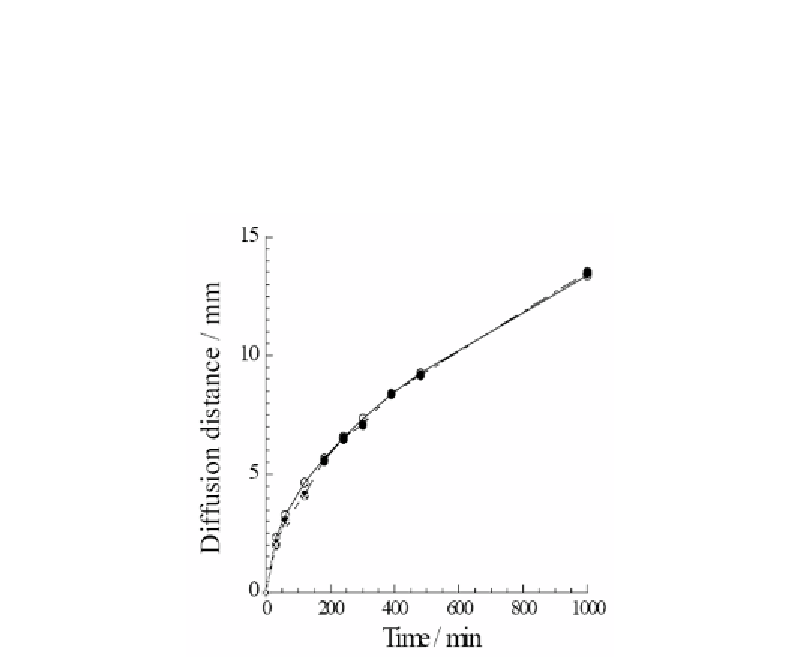
diffusion in liquid water was much quicker than that in the agarose solid. The experimental
diffusion distance of thionine in the solid as shown in figure 15 was in good agreement with
the calculated value (shown by a solid curve) based on self-diffusion, that is, only self-
diffusion contributes to the transport of thionine. These results show evidently that in the
agarose solid convection is almost entirely prohibited. It should also be noted that for
diffusion of solutes in a liquid, contribution of convection is extremely larger than self-
diffusion.
Figure 15. Diffusion distance of thionine against time in agarose solid. (-
〇
-)Theoretical value of
diffusion distance of thionine by self-diffusion according to eq.(1) [6]. (●) Observed diffusion distance
of thionine in agarose gel. In a pure liquid water thionine became homogeneous within 40 min in a
reference experiment under the similar conditions.
In recent years crystal growth in solid phases such as biomaterials, gels, and polymers is
attracting a great deal of attention [31,32]. Star-shaped calcite (CaCO
3
) crystals have been
found to be formed in agarose gels (1 wt%) different from the typical rhombohedral calcite
crystals [32]. In that work an agarose gel (1 wt%) containing CaCl
2
was soaked in an aqueous
solution of Na
2
CO
3
so that the CO
3
2-
ions diffuse into the gel to form CaCO
3
crystal. The
reason for the star-shaped calcite crystal formation was inferred to be due to the slow
diffusion of CO
3
2-
ions from the outer liquid water phase into the gels. In a sense it is true that
the diffusion of the carbonate ions from the outer liquid water phase into the gel should be
slow. On the other hand convection factor should also be taken into account for crystal
growth in matrixes. Convection disturbs the concentration gradient of solutes above a
growing crystal surface, which hinders ideal crystal growth. In the absence of convection the
solute concentration gradient above a growing crystal is not disturbed, which allows slow
solute supply and therefore slow crystal growth to form high quality single crystals, as carried
out in the cosmic space where convection does not take place due to the absence of gravity.
Search WWH ::

Custom Search