Environmental Engineering Reference
In-Depth Information
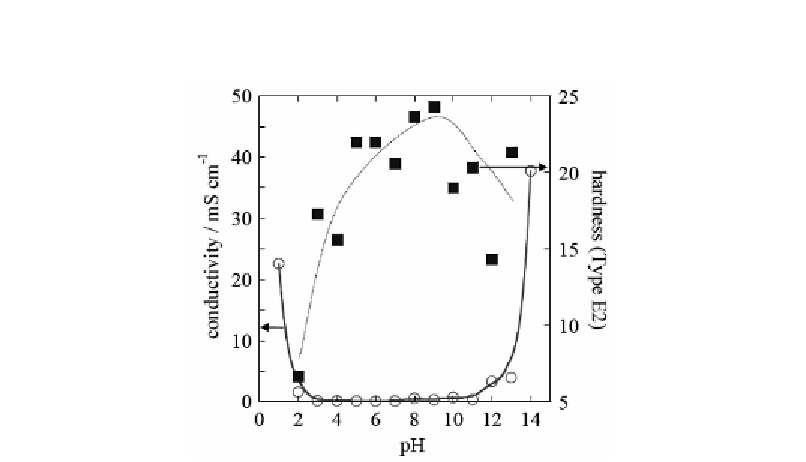
The pH dependence of the hardness and the conductivity of the agarose solids containing
0.1 M KCl are shown in figure 13.
Figure 13. pH dependence of the conductivity and hardness of 4 wt% agarose solids containing 0.1 M
KCl aqueous solution [5].
The conductivity has increased both in the strong acidic and the strong basic conditions,
which is attributed to the increase in the H
+
or OH
-
concentrations. On the other hand, the
hardness seems to show a maximum around pH 9, and decreases from neutral region towards
acidic conditions although the data show rather high degree of scattering. The agarose solid
becomes soft at low pH, because the agarose helical structure are destroyed. Thus, the
hardness of the solid is high at pH 4-10, which is ascribable to stabilization of helical
structures.
Conclusive Remarks
These results are summarized as follows. Electrochemical impedance spectrum was
measured in the polysaccharide solid showing that both the ionic conductivity of the
polysaccharide solid and the double-layer capacitance on the electrode surface are the same as
those in an aqueous solution of the ions. Impedance spectra of 1-5 wt% agarose containing
0.1 M KCl showed almost linear relations at all the frequencies but the spectra changed with
the polysaccharide concentration. From 1 to 3 wt% the conductivity decreased with the
increase in the agarose concentration, while from 3 to 5 wt% the conductivity increased with
the increase in the hardness of the agarose solid. The conductivity of the agarose solids
containing 0.1 M KCl was large both in the strong acidic and strong basic conditions, which
is attributed to the increase in the H
+
or OH
-
concentration. The conductivity of the solids was
discussed in relevant to their hardness. Thus, it was elucidated that the polysaccharide solid
containing excess water can be used as new ionically conductive solid for conventional
electrochemistry. Other many polysaccharides can be used in principle as a solid medium for
electrochemistry.
Search WWH ::

Custom Search