Environmental Engineering Reference
In-Depth Information
On cooling a hot aqueous solution, the polysaccharide chains form helical structure
which then aggregate to double helix. This double helical structure further aggregates to a
bundled structure. The double and/or bundled helical structures act as a bridging point for a
three-dimensional (3D) network that can contain excess water within the network [1,13-15].
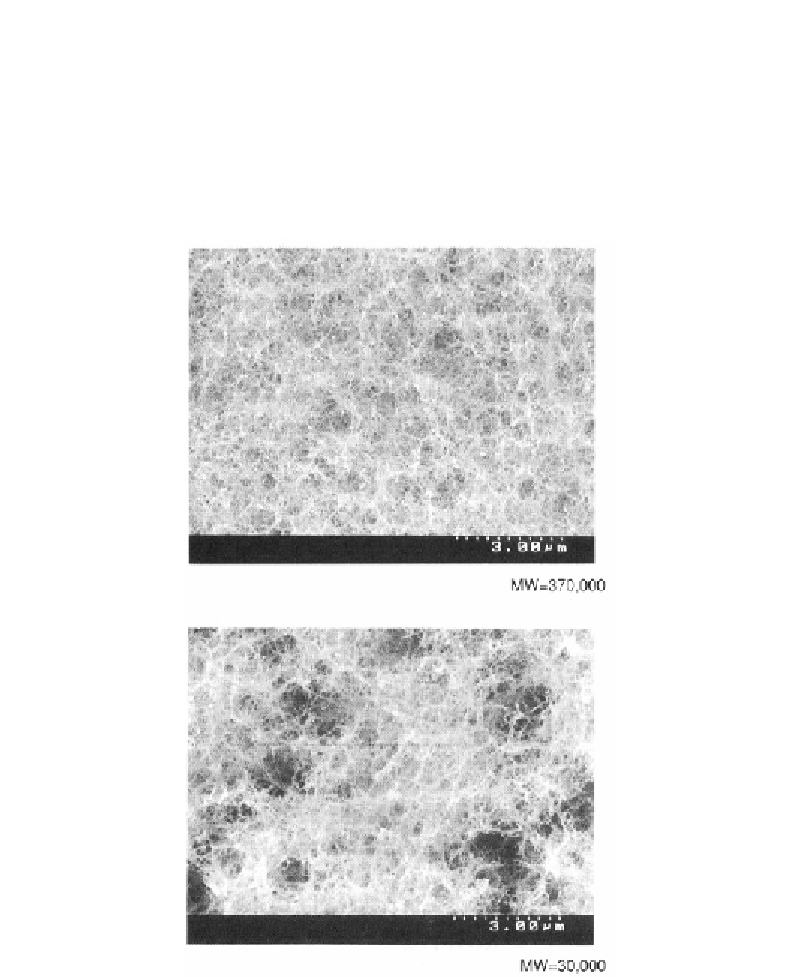
The scanning electron micrograph of agar-agar (major component is agarose) is shown in
figure 2 [14]. For the lower molecular weight sample (lower strength sample), the entangled
structures are localized and week, but for the higher molecular weight sample (higher strength
sample) the bridged network structures are delocalized and strong.
Figure 2. Scanning electron micrograph of agar-agar for higher and lower molecular weight (MW)
samples [14].
Since κ-carrageenan involves sulfonate anionic groups, the helical structures are bundled
by the presence of cations that induce aggregation of helixes by electrostatic interaction.
Very tight polysaccharide solids containing excess water could be obtained by applying
microwave very carefully when preparing its aqueous solution [2]. The hardness of
polysaccharide solids containing excess water is shown in figure 3.
Search WWH ::

Custom Search