Environmental Engineering Reference
In-Depth Information
take place [6] meaning that molecular diffusion can be discriminated from bulk convection
that should always exist on the earth due to the gravity.
Recently a dye-sensitized solar cell is attracting a great deal of attention to convert solar
energy into electricity [7]. Since this cell uses redox electrolyte solution, it is of importance to
solidify the liquid in order to stabilize the cell, but the task is not easy to achieve. To
overcome this problem solidification of the organic redox electrolyte solution by molten salts
and gelator [8] or by polymer film [9,10] has been achieved.
We have successfully used the
polysaccharide solid to solidify the electrolyte solution [11,12].
In the present review the fundamental properties of polysaccharide solids containing
excess water are at first explained (section 2). In the section 3 the characteristics of the
polysaccharide solids as media for electrochemistry will then be described in detail. A novel
property of the polysaccharide solid will be shown wherein bulk convection does not take
place (section 4). The successful application of the polysaccharide solid to solidify the redox
electrolyte solution of a dye-sensitized solar cell will be introduced in section 5 followed by
future scopes of the solid medium (section 6).
Properties of Polysaccharide Solids Containing Excess Water
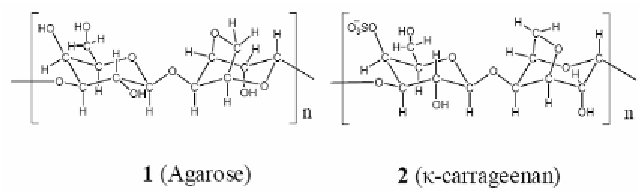
The typical polysaccharides reported in the present review are agarose (1) and κ-
carrageenan (2). It has well been known that polysaccharides form a tight and elastic solid
containing excess water [1,13-15].
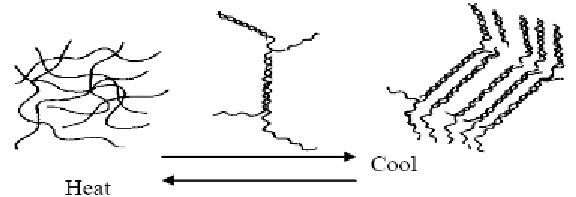
Gelation mechanism of agarose, for an example, is shown in figure 1 [14].
Figure 1. Gelation mechanism of agarose [14]:On cooling a hot solution, the chains form helical
structures which then aggregate to double helix, further aggregating to a bundled structure. The double
and/or bundled helical structures act as a bridging point for 3D network structure.

Search WWH ::

Custom Search