Environmental Engineering Reference
In-Depth Information
This result is attributable to tendency of the ensuing electro-
chemical reactions that reduce Cr(VI) to Cr(III), which sub-
sequently is adsorbed onto the clay surfaces and no longer
available for transport.
F.
Sulfate, SO4-2, Nitrate, NO3-, and Chloride, Cl- extraction
from cationic/anionic soils
:
Simultaneous extraction of multiple anionic compounds
introduced as lead salts was tested in anionic (positive sur-
face charge) and cationic (negative surface charge) clayey
soils. Mixed with medium sand, the clay substrates were
predominantly kaolinite (cationic - PZC: 2-4.6) and gibbsite
(anionic - PZC:10). The test specimens were prepared by
spiking with Pb(SO
4
); Pb(NO
3
)
2
, and PbCl
2
salt solutions
at their respective solubility limits. Electrokinetic tests were
conducted over 24 hours, at the end of which the distribu-
tions of the anions were measured in the electrode cham-
ber solutions and across the soil specimens at five points.
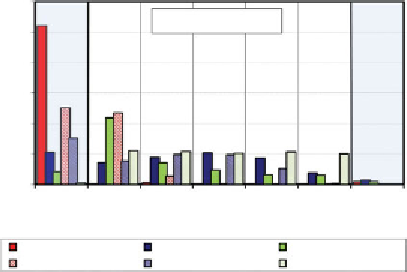
Figure 2.22 shows this distribution for the three anions in
two different clay types. As observed, the extraction appears
to be influenced both by the anion and the type of sub-
strate. The anions of the soluble salts were extracted into
the anode more effectively that the less soluble ones. The
cationic soil appeared to release the anions more effectively
than the anionic soil, as the anions would have higher affin-
ity to the clay surface in the latter case. Figure 2.23 shows the
mass fraction of anions extracted to anode per Coulomb of
1.2
Test Duration = 24 hrs
1
0.8
0.6
0.4
0.2
0
ANODE
0 0.25
Normalized distance from anode end
0.5
0.75
1
CATHODE
Cationic Soil/NO3
Anionic Soil/NO3
Cationic Soil/Cl
Anionic Soil/Cl
Cationic Soil/SO4
Anionic Soil/SO4
Figure 2.22
Post-EK distribution of mass fraction of anions of lead salts in cationic and
anionic clay soils















Search WWH ::

Custom Search