Environmental Engineering Reference
In-Depth Information
(+)
(-)
d
ρ
∞
ψ
s
ρ
s
ρ
s
ψ
s
ψ
∞
d
2
d
2
h=
h=0
h=
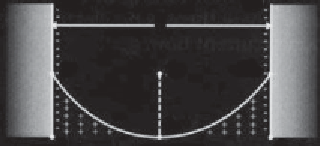
Figure 1.5
Two charged surfaces separated by distance
d
with a fluid in between. The film
thickness on each surface is
h
=
d
/2. The number density of the counter ions at the surface
is
ρ
s
and the center is designated
ρ
∞
,
which is taken as zero at the reference point in the
center. The electric field, which is independent of distance is equal to the electric charge
density,
D
, divided by the electric permittivity,
E
s
= D/εε
o
(after Donaldson and Alam,
2008).
In aqueous solutions, the zeta potential of mineral surfaces is a function of
pH. Usually, acidic solutions promote positive charges at the surface result-
ing in a positive zeta potential, whereas basic solutions produce an excess
of negative charges at the surface because of increase in the hydroxyl ion
concentration. The
pH
at which the zeta potential is equal to zero is defined
as the zero point charge
(zpc).
When the negative and positive charges of
ions in a solution are equally balanced, the solution is electrically neutral
and this condition is defined as the isoelectric point
(iep)
(Donaldson and
Alam, 2008).
Thomson and Pownall (1989) observed an approximate linear
trend of the zeta potential with respect to pH for calcite in dilute solutions
of sodium chloride and a mixed solution of sodium chloride and sodium
bicarbonate, where
ζ = -
6.67
*pH +
40. The zero point charge occurred at
pH >
6. Sharma et al. (1987) reported an inverted S-shaped trends where
ζ = -
20
*pH +
100
(zpc
at
pH ≈
5
)
for Berea Sandstone cores and dilute
sodium chloride solutions (see Donaldson and Alam, 2008).
As the electrolyte passes through a porous material (rock, glass, capillar-
ies, etc.), a potential difference develops which is usually called the stream-
ing potential. The expression for the streaming potential can be written in
terms of the zeta potential and the combined resistivities of the electrolyte
and solid (Kruyt, 1952):
⎡
⎛
⎜
2
⎞
⎤
d
dx
y
ez
pm
R
C
Nm
1
Vsm
C
V
Pa
⎛
⎜
⎞
⎟
(
)
⎜
⎞
⎟
⎛
⎞
⎟
=
0
V
⎟
=
(1.4)
⎢
⎜
⎥
4
2
Pa s
.
⎣
⎦
where
x
is the distance;
R
0
is the combined resistivities of the electrolyte
and solid; and
μ
is the viscosity.



















Search WWH ::

Custom Search