Environmental Engineering Reference
In-Depth Information
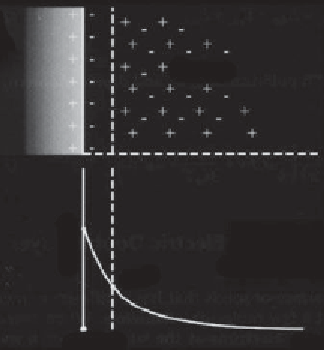
LIQUID
SOLID
ψ
S
ψ
S
= potential at the solid surface
= zeta potential, potential at the shear plane
= potential in the bulk liquid (=0)
ψ
ζ
ψ
∞
ψ
ζ
x
s
x
ς
x
∞
Figure 1.2
Electric double layer at the interface between a solid and liquid:
x
s
= surface of
the solid,
x
ζ
= shear plane,
x
∞
= bulk liquid,
x
ζ
- x
s
= stern layer,
x
∞
- x
ζ
= electrical diffuse
(Gouy) layer (Debye length,
1/ κ
) (after Donaldson and Alam, 2008).
plane and the bulk fluid is known as the zeta potential (Donaldson and
Alam, 2008) (see Fig. 1.3).
Cations, anions, and molecules with electrical dipoles can be
adsorbed by nonelectrical forces. Grahame (1947) observed that anions
are adsorbed by nonelectrical forces with the centers of negative charges
lying on an inner plane (within the Stern layer) from the surface known
as the inner Helmholtz plane (
IHP
at
x
i
distance from the solid sur-
face, Fig. 1.4). The
IHP
is followed by the outer Helmholtz plane (
OHP
)
drawn through the charges of the hydrated counterions.
The thickness of the Helmholtz layers thus reflects the size of the
adsorbed anions and counterions within the Stern layer and is observed by
the differences of the measured linear potential differences within the Stern
layer. An excellent discussion on the subject was presented by Donaldson
and Alam (2008).
The length of the exponential electrical field decay (from the shear plane
to the bulk fluid) is known as the Debye length (1
/κ
). For example, if the
plates of a capacitor have equal charge densities, the zeta potential is the
potential difference from the center of the separation to one of the plates
(Donaldson and Alam, 2008):
1
2
=
⎛
⎞
⎟
2
2
1
ee y
s
ee
r
kT
ze
⎡
⎢
C
Jm
J
C
m
C
⎤
⎥
m
(1.2)
=
os
oB
=
⎜
22
k
c
i


















Search WWH ::

Custom Search