Environmental Engineering Reference
In-Depth Information
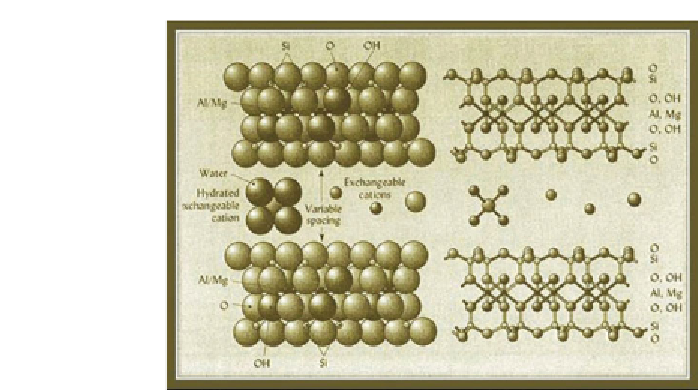
Figure 3.11
Montmorillonite Crystal structure Showing Interlayer Water and
Exchangeable Cations (after UBC Website)
• Electro-osmosis
• Induced polarization
Normal Effect
in shaly-sands
Shaly sands with high Q
v
, tend to have lower permeability than those with
low Q
v
.
3.8.5
Electrochemistry of the Double Layer
Water is such a strong polar molecule that it preferentially (over non-polar
and weakly polar petroleum molecules) wets the clay mineral surfaces,
making them hydrophilic, or preferentially water-wet. The bound layer of
water molecules is held tightly enough by the electrostatic attraction from
the excess negative charges in the clay mineral surfaces that these water
molecules do not move under normal sub-surface forces.
When water comes in contact with clay, or another phillosilicate, the
water molecules closest to the surface align themselves with their posi-
tive (hydrogen ion) pole opposite the negative charges on the surface of
the silicate minerals (figures 3.10 and 3.12). This region of water mole-
cules (which may be from a single-molecule to several-molecules thick)
is tightly bound to the surface of the clay minerals, and is variously called
the “bound layer”, “Stern layer”, or “inner Helmholtz layer”. The inner layer
may or may not contain cations from ionic salts in solution but
will not
contain any anions
.














Search WWH ::

Custom Search